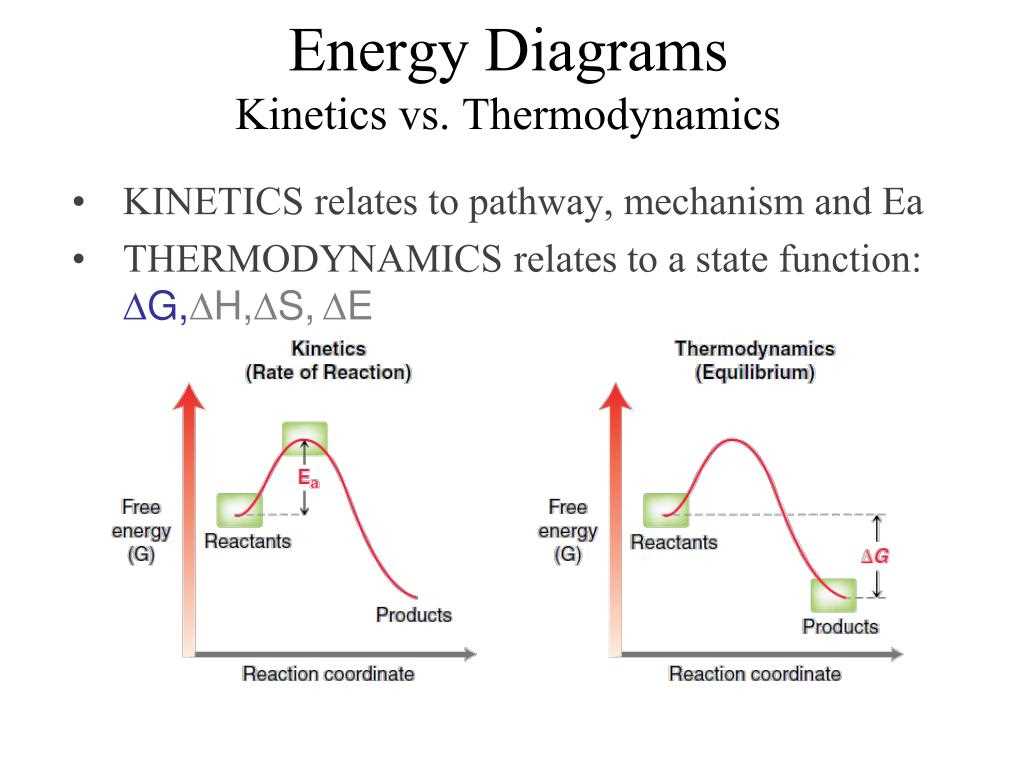
Thermodynamics is a branch of physics that deals with the relationships between heat, work, and energy. It is a highly specialized field with many different concepts and diagrams that help engineers and scientists understand and analyze thermodynamic systems.
One of the most commonly used diagrams in thermodynamics is the Ts diagram, also known as the temperature-entropy diagram. This diagram is used to represent the thermodynamic states of a substance as it undergoes various processes.
In a Ts diagram, the vertical axis represents temperature, while the horizontal axis represents entropy. Temperature is a measure of the average kinetic energy of the particles in a substance, while entropy is a measure of the amount of energy in a system that is unavailable to do work.
By plotting the thermodynamic states of a substance on a Ts diagram, engineers and scientists can analyze the efficiency of different processes, determine the potential for work, and understand how energy is transferred and transformed within a system. The Ts diagram is an invaluable tool in the field of thermodynamics and is used in a wide range of applications, from power plant design to refrigeration and air conditioning systems.
What is a TS Diagram in Thermodynamics?
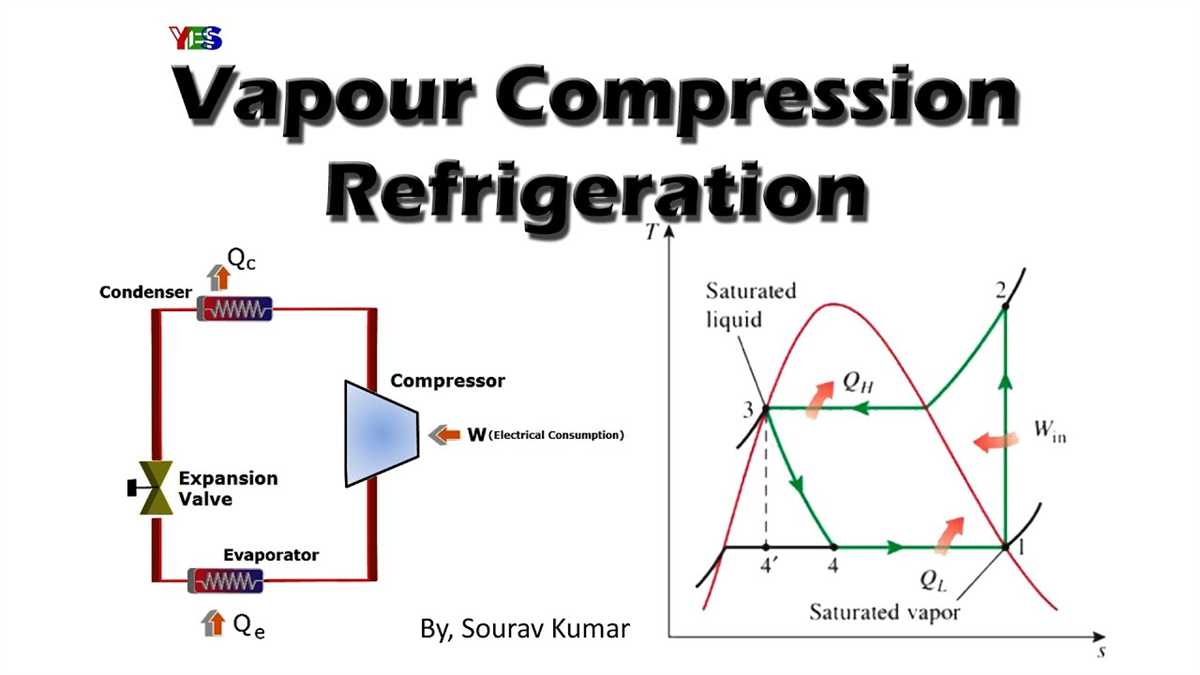
A TS diagram, also known as a T-s diagram or temperature-entropy diagram, is a graphical representation of the thermodynamic processes that occur in a system. It is commonly used in thermodynamics to visualize the changes in temperature and entropy of a substance or system during various processes.
The TS diagram consists of two axes: the horizontal axis represents temperature and the vertical axis represents entropy. The shape and position of the curves on the TS diagram provide valuable insights into the behavior and efficiency of a system.
The T-s diagram is particularly useful in analyzing and understanding the performance of thermodynamic cycles and processes, such as the Carnot cycle or the Rankine cycle. It allows engineers and scientists to evaluate the efficiency and work potential of different systems by examining the paths and transitions on the diagram.
On a T-s diagram, various processes such as compression, expansion, heating, and cooling can be represented by distinct curves or lines. For example, an isobaric process is represented by a horizontal line on the diagram, indicating constant pressure, while an isothermal process is shown as a vertical line to indicate constant temperature.
In addition to visualizing processes, the T-s diagram can also assist in analyzing the properties of substances at different states or points. By considering the slopes of the curves, one can determine the specific heat capacities and the behavior of the substance during phase changes.
Overall, the T-s diagram is an essential tool in thermodynamics that helps engineers, scientists, and students understand and analyze the behavior and efficiency of thermodynamic systems. It provides a visual representation of the processes occurring in a system and allows for a comprehensive analysis of their properties and performance.
Definition and Explanation
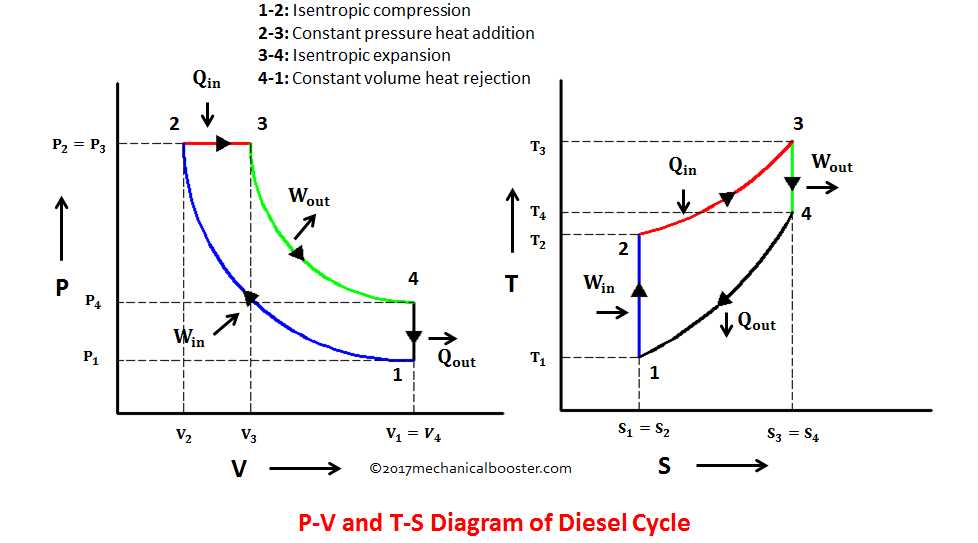
The T-s diagram, also known as the temperature-entropy diagram, is a graphical representation of the thermodynamic properties of a substance. It is commonly used in thermodynamics to visualize and analyze processes and cycles in a system. The T-s diagram plots temperature on the horizontal axis and entropy on the vertical axis.
In thermodynamics, temperature represents the average kinetic energy of the particles in a substance, while entropy measures the degree of disorder in a system. The T-s diagram allows engineers and scientists to analyze how these properties change throughout a process and understand the behavior of a substance.
On the T-s diagram, lines of constant pressure, volume, and quality can be plotted to represent different states or conditions of a substance. The shape and position of these lines provide important information about the thermodynamic characteristics of the substance.
The T-s diagram is particularly valuable for analyzing and designing thermodynamic cycles, such as the Rankine cycle in power plants or the refrigeration cycle in cooling systems. By examining the T-s diagram, engineers can optimize the efficiency of these systems and make informed decisions about their design and operation.
In conclusion, the T-s diagram is a powerful tool in thermodynamics that allows for the visualization and analysis of thermodynamic properties. It provides valuable insights into the behavior of substances and helps engineers and scientists optimize the performance of thermodynamic processes and cycles.
Key Features and Components
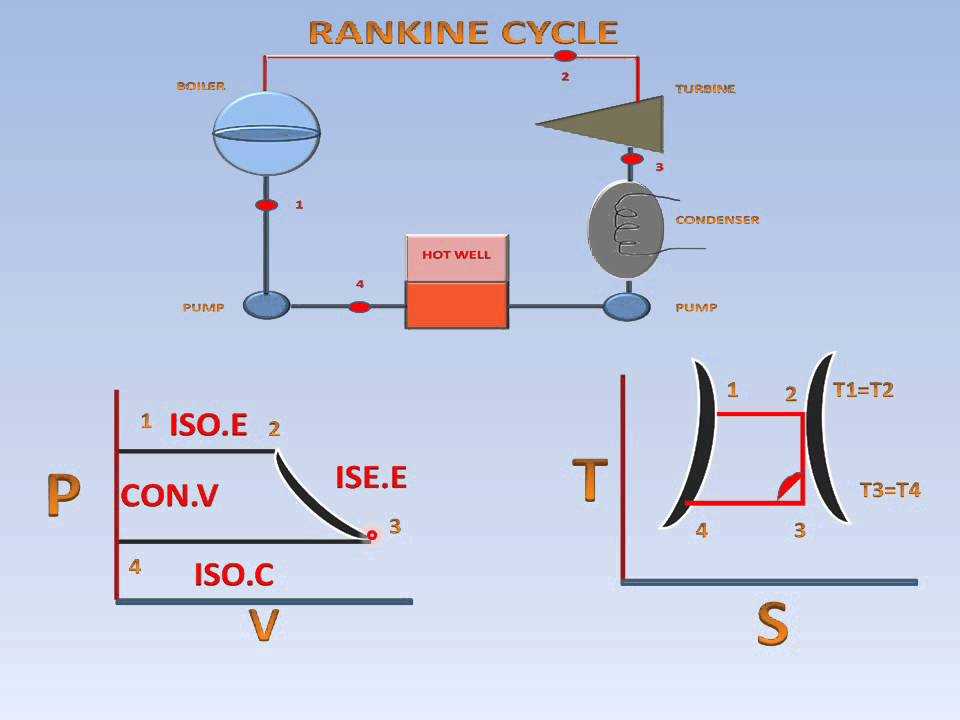
The T-s (temperature-entropy) diagram, also known as the Mollier diagram, is a graphical representation commonly used in thermodynamics to analyze and visualize the behavior of a substance during a thermodynamic process.
The T-s diagram consists of two axes – the temperature axis (T) and the entropy axis (s). The temperature axis is usually horizontal, while the entropy axis is vertical. The diagram is divided into different regions that represent different phases and states of the substance.
One of the key features of the T-s diagram is the saturation curve, which represents the boundary between the liquid and vapor phases of a substance. It shows the conditions under which the substance exists as a pure liquid, a pure vapor, or a mixture of both.
Another important component of the T-s diagram is the critical point. This point represents the temperature and pressure at which the substance undergoes a phase change from a liquid to a vapor, without any distinct boundary between the two phases. The critical point is the highest temperature and pressure at which a substance can exist as a vapor.
The T-s diagram also includes lines of constant pressure, constant volume, and constant entropy. These lines, known as isobaric, isochoric, and isentropic lines, respectively, provide valuable information about the behavior and properties of the substance during a thermodynamic process.
In addition, the T-s diagram can be used to analyze various thermodynamic processes, such as isothermal, adiabatic, and reversible processes. It helps to understand the changes in temperature, pressure, and entropy during these processes and provides insights into the efficiency and performance of thermodynamic systems.
Overall, the T-s diagram is a powerful tool in thermodynamics that allows engineers and scientists to visually represent and analyze the behavior of substances during thermodynamic processes. Its key features and components provide valuable insights and understanding of the thermodynamic properties and behavior of substances.
Importance of TS Diagrams in Thermodynamics
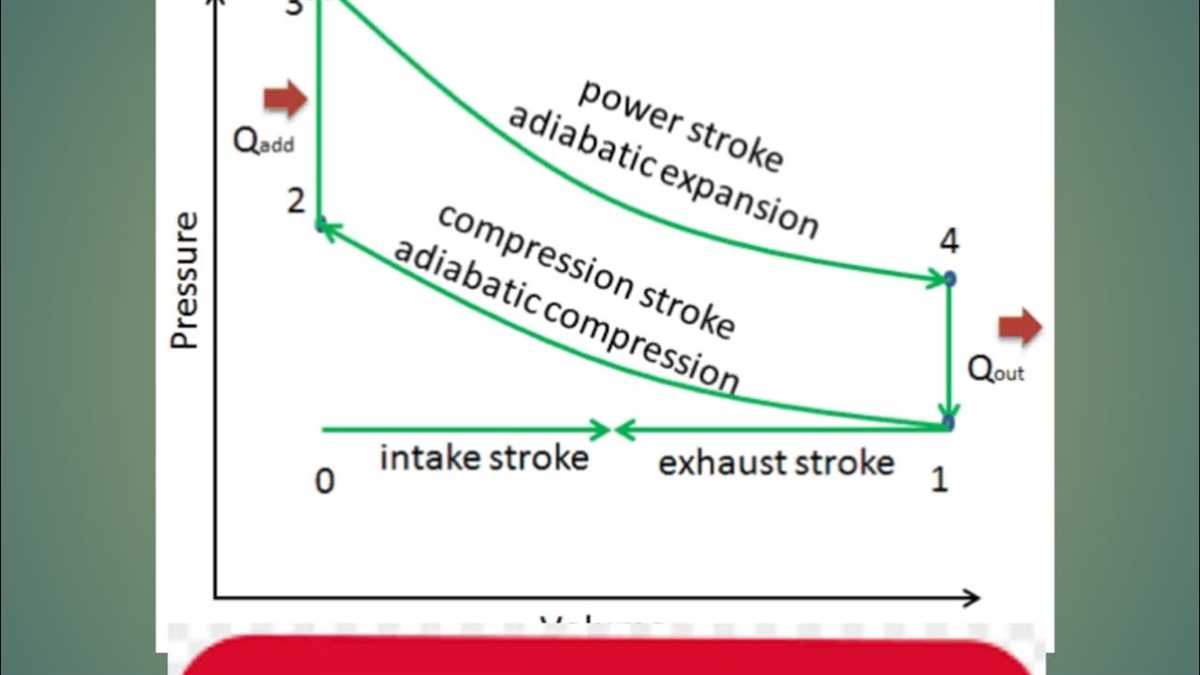
The TS diagram, also known as the temperature-entropy diagram, is a graphical representation that is widely used in thermodynamics to analyze and visualize the behavior and properties of a substance or system. It plots temperature on the vertical axis and entropy on the horizontal axis. TS diagrams provide valuable insights into the thermodynamic processes and help engineers and scientists in various fields to understand and optimize their systems.
One of the key advantages of TS diagrams is that they allow for a visual representation of complex thermodynamic processes, making it easier to analyze and understand the behavior of a system. By plotting the various states and paths of a substance on the TS diagram, engineers can quickly identify inefficiencies, deviations from ideal behavior, and areas for improvement.
TS diagrams also play a crucial role in determining the suitability and efficiency of different thermodynamic cycles and processes. By plotting the ideal and actual cycles on a TS diagram, engineers can evaluate the Carnot efficiency and the efficiency of actual processes, such as the Rankine cycle in power plants or the Brayton cycle in gas turbines. This helps in the design and optimization of these systems to maximize efficiency and minimize energy losses.
Furthermore, TS diagrams are used to analyze phase transitions and equilibrium conditions. For example, in the case of steam, the TS diagram allows us to determine the conditions under which water would exist as a liquid, vapor, or a combination of both. This information is crucial in designing and operating steam power plants and other systems that utilize phase changes.
In conclusion, TS diagrams are powerful tools in thermodynamics that aid engineers and scientists in understanding, analyzing, and optimizing thermodynamic systems. They provide a visual representation of complex processes, allow for the evaluation of efficiency, and help in analyzing phase transitions and equilibrium conditions. Overall, understanding and interpreting TS diagrams are essential skills for anyone working in the field of thermodynamics.
Applications and Examples
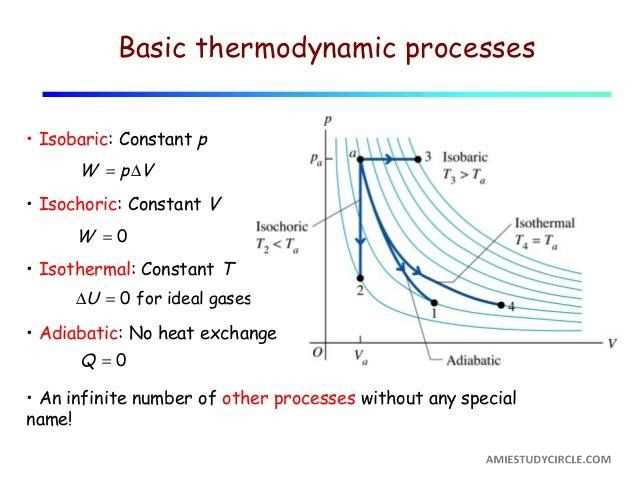
The T-s diagram is widely used in thermodynamics to analyze and visualize the behavior of substances as they undergo different processes and transformations. It provides a graphical representation of the thermodynamic properties of a substance, such as temperature and entropy, as it moves between different states.
One of the common applications of the T-s diagram is in the analysis of power cycles, such as the Rankine cycle used in power plants. The T-s diagram helps engineers understand and optimize the efficiency of the cycle by visualizing the changes in temperature and entropy at each stage of the cycle.
The T-s diagram is also used in refrigeration and air conditioning systems. It helps in understanding the performance of these systems by visualizing the compression and expansion processes and identifying areas of inefficiency. By analyzing the T-s diagram, engineers can make adjustments to improve the efficiency and performance of the systems.
Another application of the T-s diagram is in the analysis of steam turbines and compressors. The diagram helps engineers understand the thermodynamic behavior of the working fluid as it passes through the turbine or compressor. By studying the T-s diagram, engineers can optimize the design and operation of these devices to achieve higher efficiency and performance.
In addition, the T-s diagram is used for the analysis of various thermodynamic cycles, such as the Carnot cycle, Otto cycle, and Brayton cycle. These cycles are crucial in understanding the performance of engines and power generation systems. The T-s diagram allows engineers to analyze and compare the efficiency and potential improvements of different cycles.
In summary, the T-s diagram is a powerful tool in thermodynamics for analyzing and visualizing the behavior of substances and systems. It finds applications in power plants, refrigeration systems, turbines, compressors, and the analysis of thermodynamic cycles. By utilizing the T-s diagram, engineers can optimize the efficiency and performance of these systems, leading to improved design and operation.
How to Read and Interpret a TS Diagram
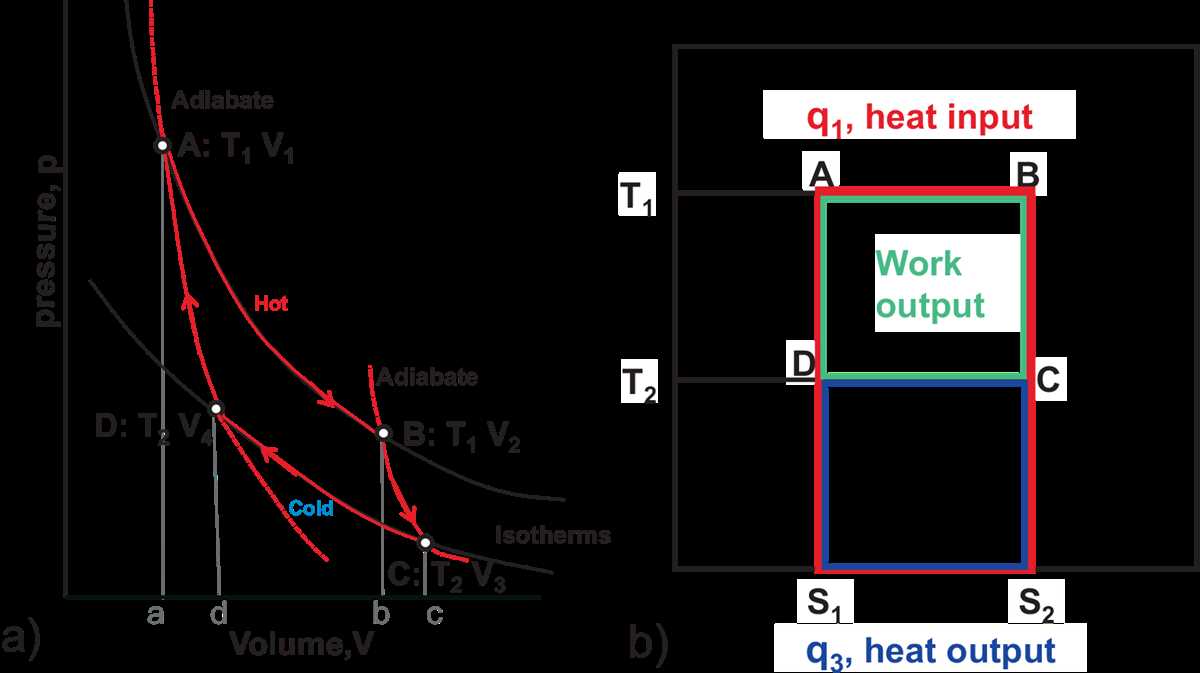
In order to effectively use and interpret a TS diagram, it is important to understand the different regions and lines that appear on it. The following steps outline a general approach to reading and interpreting a TS diagram:
Step 1: Identify the Regions
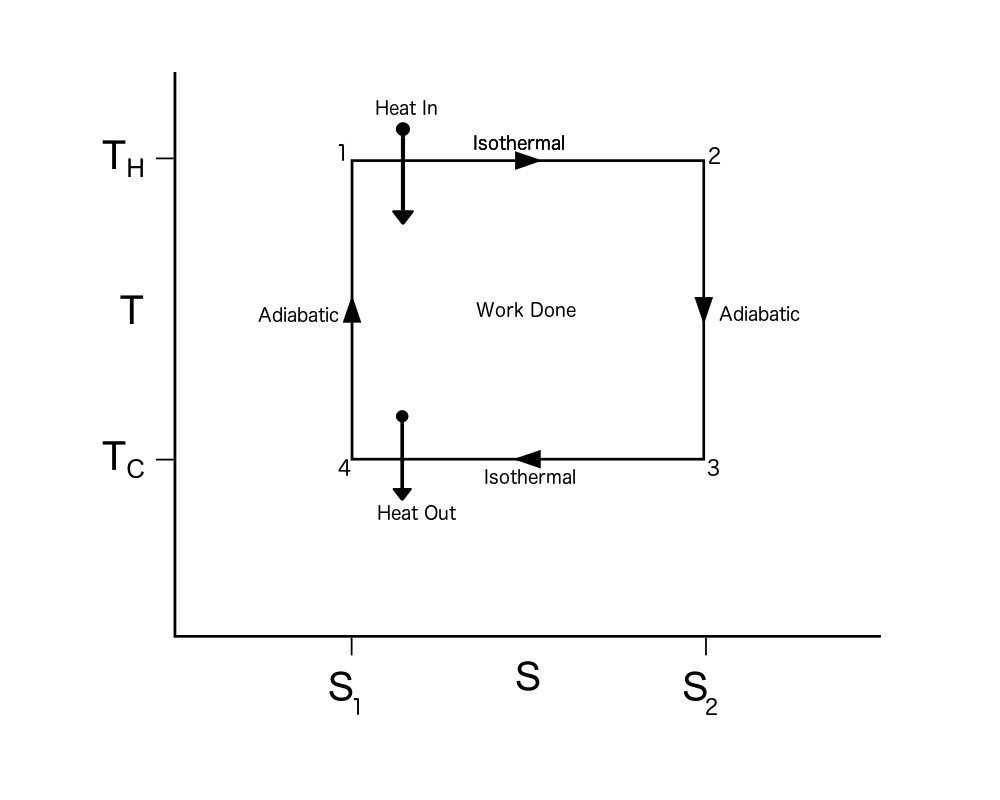
TS diagrams are divided into different regions that represent various phases or processes. The most common regions include the saturation region, the superheated vapor region, and the compressed liquid region. By identifying these regions, you can determine the state of the substance at any given point on the diagram.
Step 2: Understand the Lines
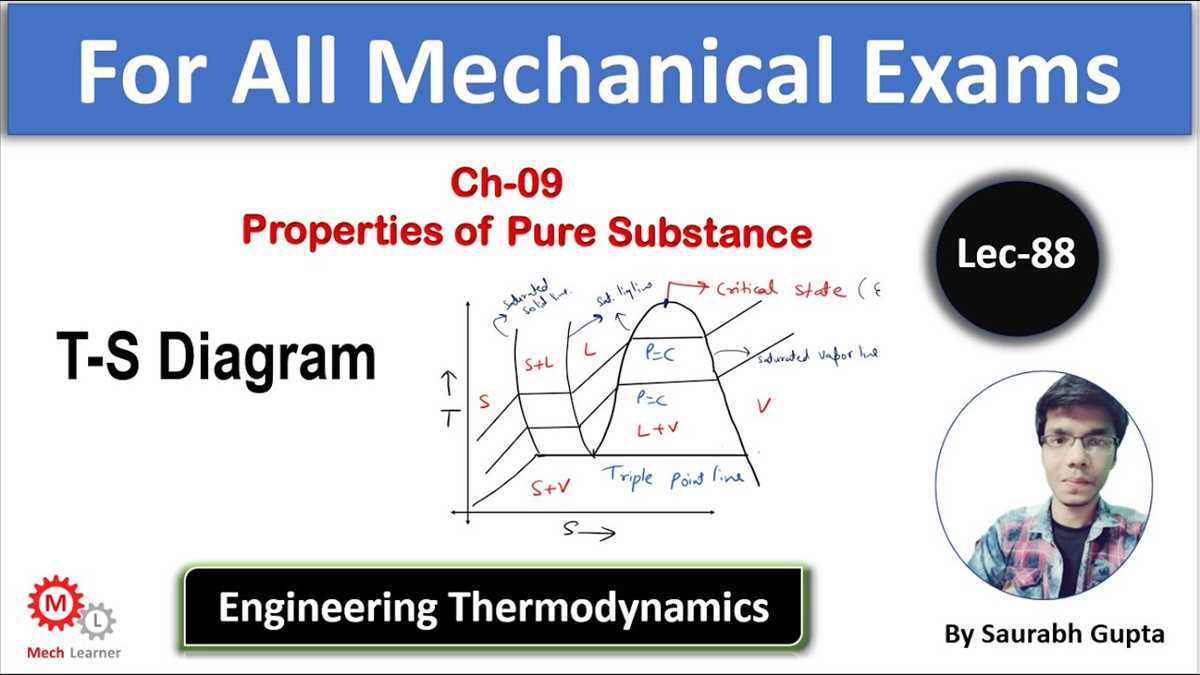
TS diagrams also include various lines that provide important information about the substance. The most common lines include:
- Saturation line: Represents the phase change between the liquid and vapor phases. The points on this line indicate the saturation temperature and pressure.
- Constant pressure lines: These lines represent constant pressure values. They can help determine the state of the substance at a specific pressure.
- Constant temperature lines: These lines represent constant temperature values. They can help determine the state of the substance at a specific temperature.
- Isentropic lines: These lines represent constant entropy values. They can help determine the efficiency of a process.
Step 3: Analyze Specific Points
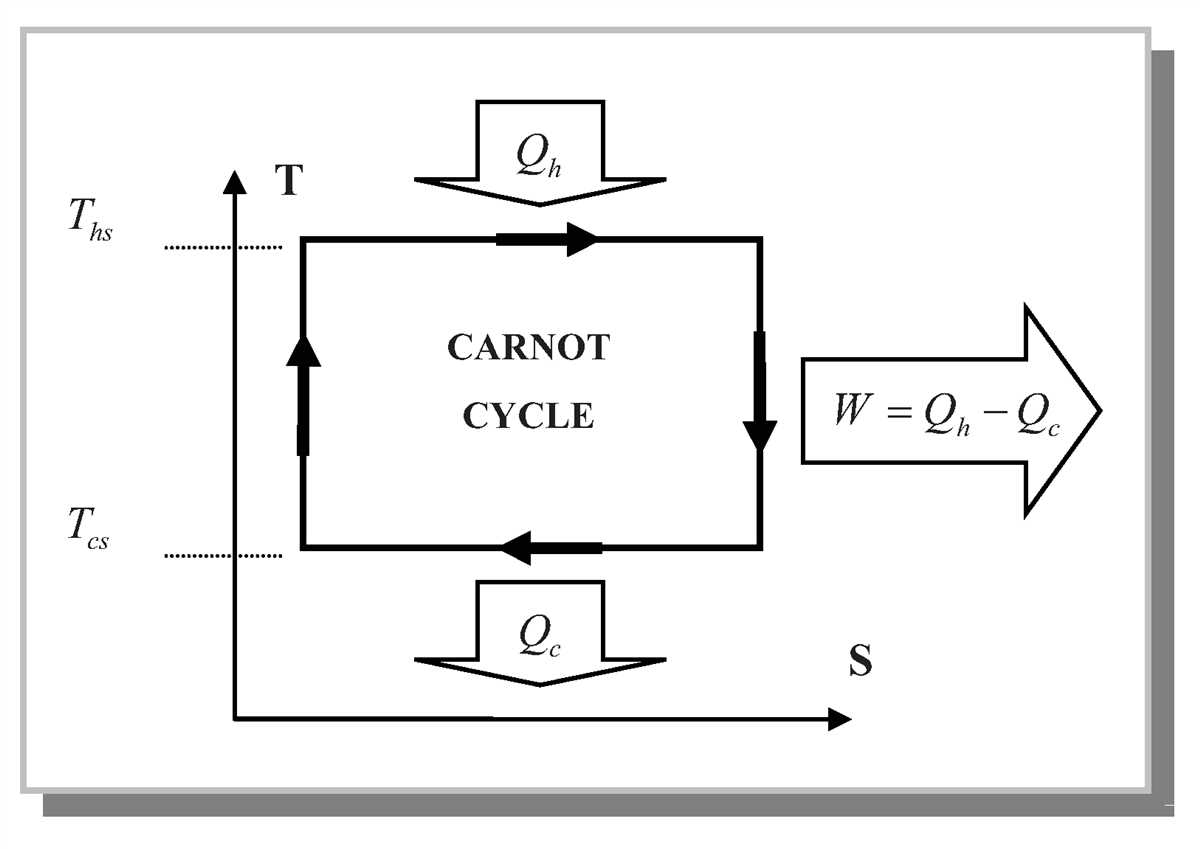
Once you understand the regions and lines on the TS diagram, you can analyze specific points to gather information about the substance or the process. By locating a point on the diagram, you can determine the temperature, pressure, specific volume, and quality of the substance at that point.
Step 4: Interpret the Process
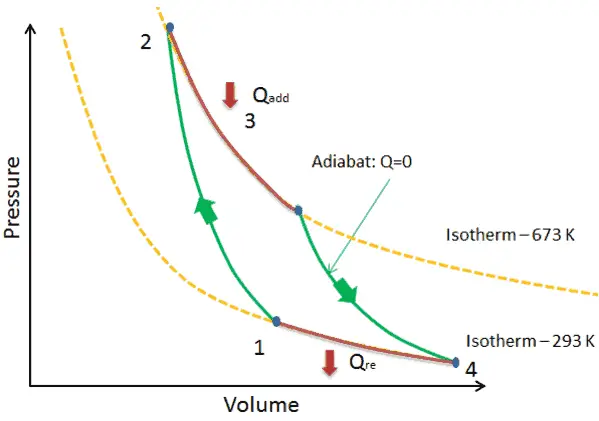
By analyzing the path or process that a substance follows on the TS diagram, you can interpret the thermodynamic behavior of the system. For example, a process that follows a horizontal line on the diagram indicates a constant temperature, while a vertical line represents a constant pressure.
By following these steps and understanding the regions, lines, and specific points on a TS diagram, you can effectively read and interpret the diagram to gain valuable insights into the thermodynamic behavior of a substance or a process.