Let’s start with an element such as Carbon. C has 4 valence electrons on it’s outer shell.
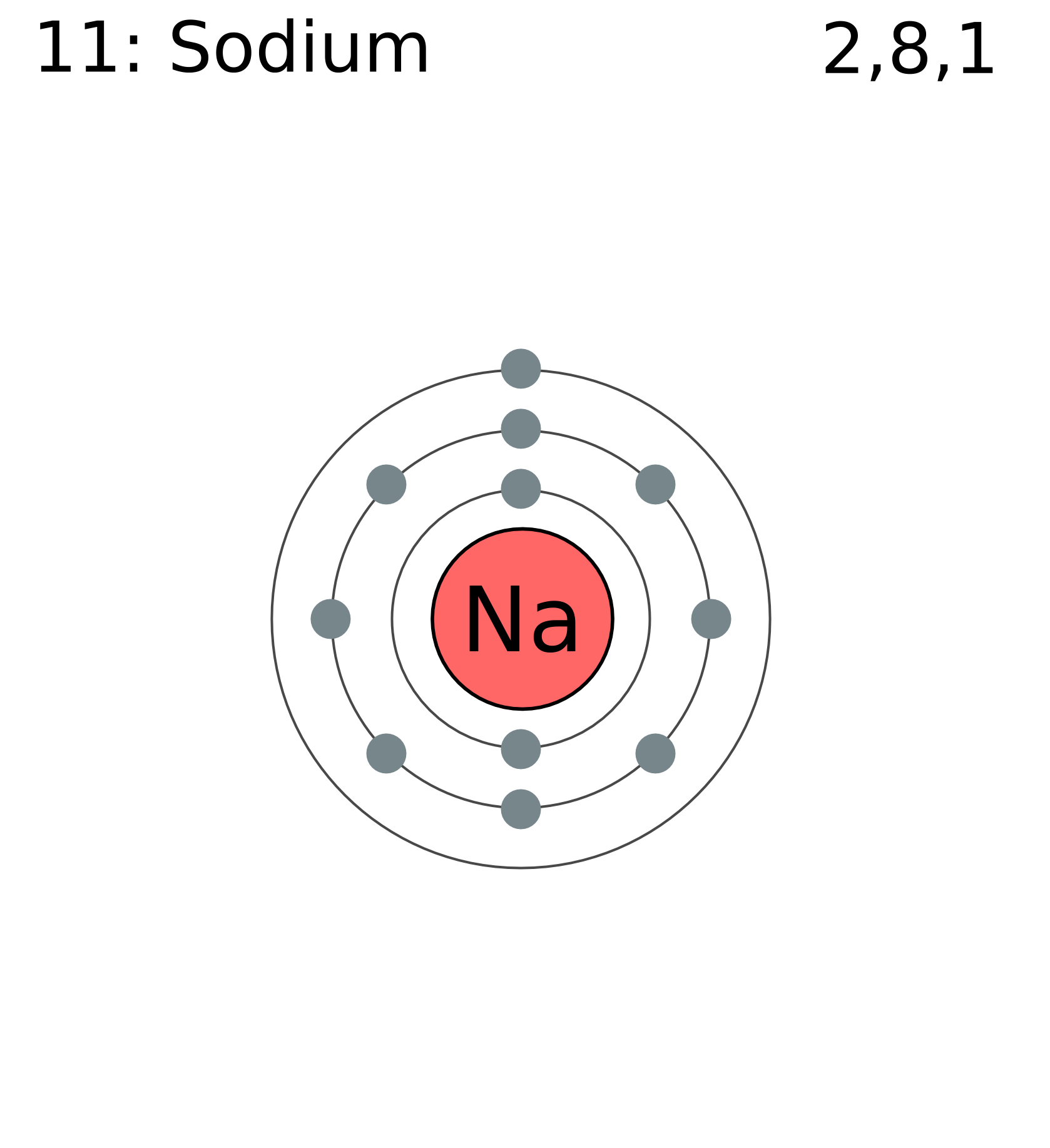
To draw C simply put the letter C in the middle and draw 4 dots around it. Uranium at Chemical schematron.org Basic Information | Atomic Structure | Isotopes | Related Links | Citing This Page Number of Protons/Electrons: Uranium 2,8,18,32,21,9,2.
U. Uranium 2,8,18,32,21,9,2.
Lewis Dot Diagrams of Selected Elements
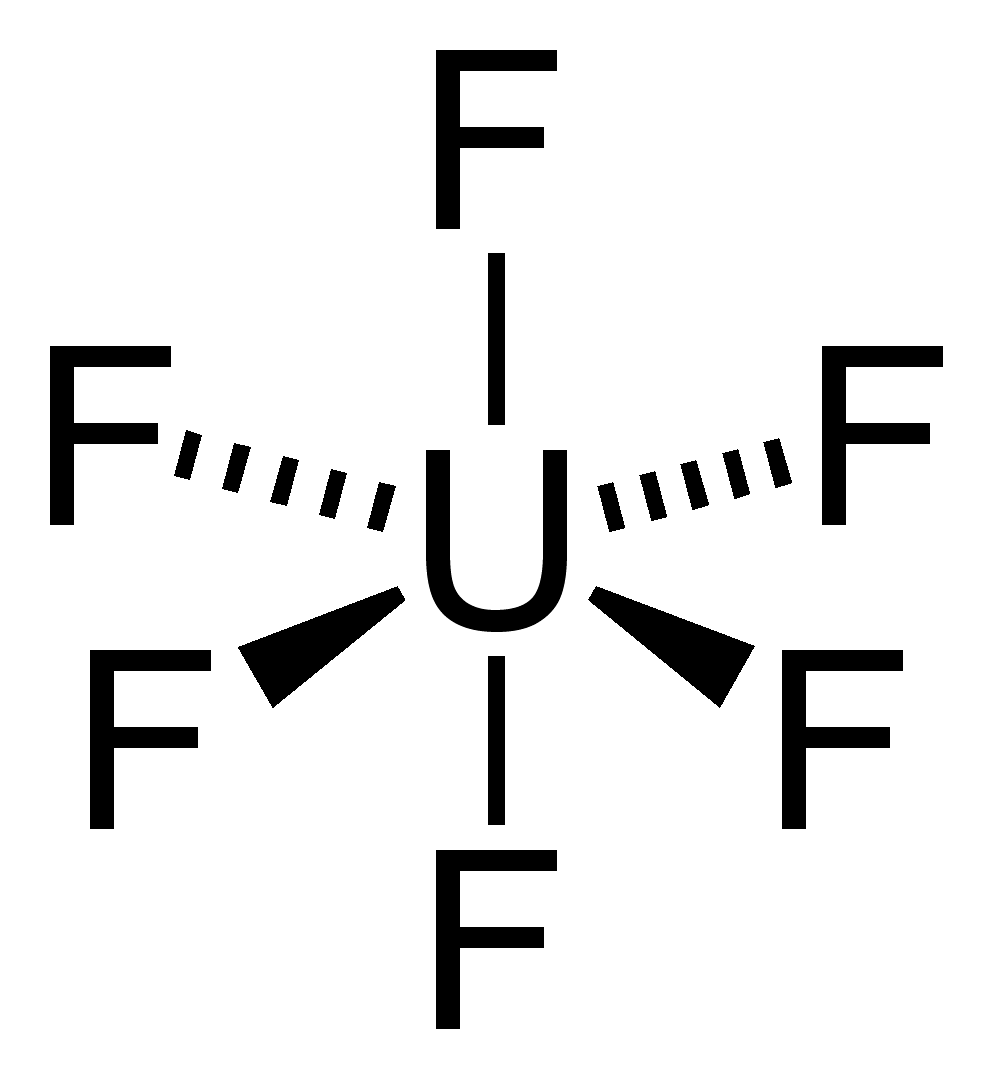
U. Uranium (U) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, Lewis Dot Diagram of Uranium (U).The electron configuration for Uranium (U) is based upon the placement of uranium in the fourth column of the actinide series or f block.
Therefore th electron configuration for uranium must end as #5f^4#, #1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 5s^2 4d^10 5 p^6 6s^2 4e^14 5d^10 6p^6 7s^2 5f^4#. Uranium (U) has an atomic mass of Find out about its chemical and physical properties, states, energy, electrons, oxidation and more. Uranium, which is a member of the uranium series (the decay chain of uranium), decays to lead through a series of relatively short-lived isotopes.
Uranium is made from thorium by neutron bombardment, usually in a nuclear reactor, and U is also fissile. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
As electrons are added, they fill electron shells in an order determined by which configuration will give the lowest possible energy. There are two types of diagrams one is the Lewis diagram the other is the Electron dot diagram. To make the electron dot diagram you put the electron symbol and put a dot on o ne of the sides for each period (you don’t count the middle section.)Ne: = Neon dot diagram ‘ ‘.File:Electron shell schematron.org – Wikimedia CommonsPeriodic Table of Elements: Uranium – U (schematron.org)