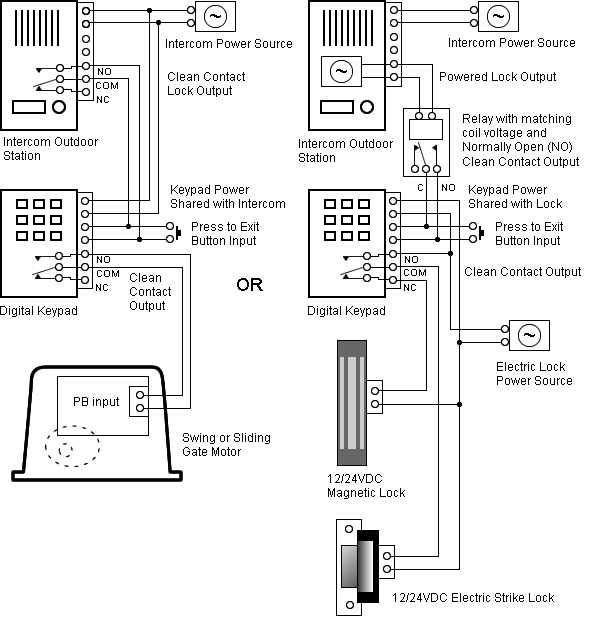
Electronic configuration of Homonuclear Diatomic Molecules.
1)H2+. Molecular orbital energy level for H2+.
The electronic configuration of H2+. Electronic configuration of Homonuclear Diatomic Molecules.
1)H2+. Molecular orbital energy level for H2+.
The electronic configuration of H2+. Answer to Create an MO diagram for H2+ H2 and H Post the Lumo, lumo -, homo, homo + near its energy level.
σ bonding MO that is lower in energy than the constituent 1s AOs and an antibonding σ* MO that is at a higher energy than the 1s AOs.[1] Each. The molecular orbital energy level diagrams for H2, H2.
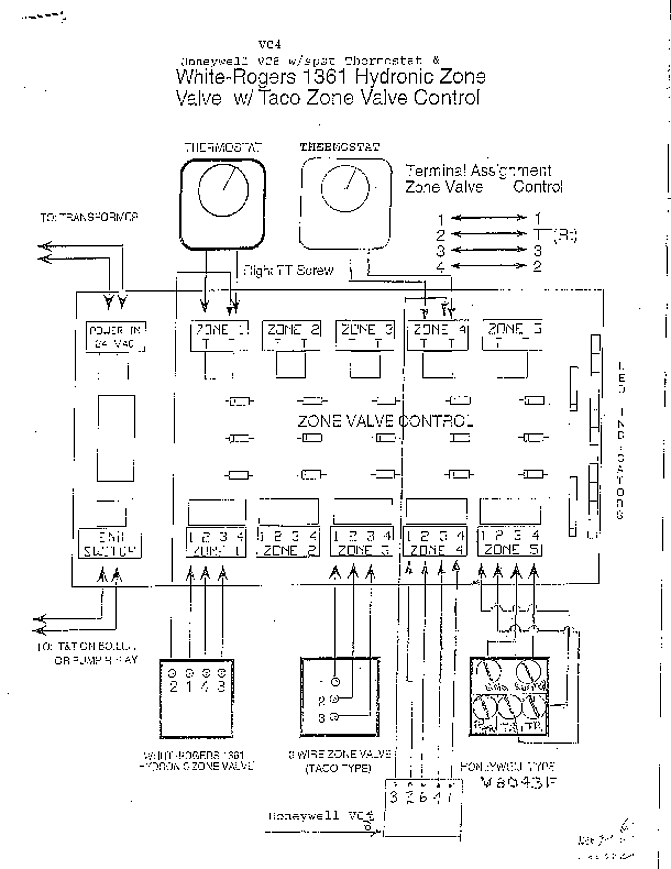
+, H2. – and O2 are shown below.
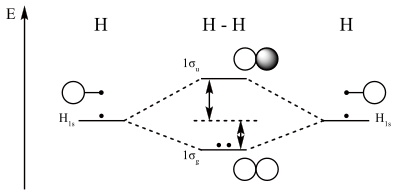
Fill in the valence electrons for each species in its ground state and.Nov 02, · For the ion H2+: a) Draw the molecular orbital diagram. b) Calculate the bond order.
c) Would this ion exist? Work, Energy, and Power: Crash Course Physics #9 – . Problem: Draw MO energy diagrams for the molecular ions H2+ and H Which ion has the stronger H-H bond?
Problem 1 Since both molecular ions have a bond order of 1/2, they are approximately equally stable. Problem: Surprisingly, the hybridization of the starred oxygen in the following molecule is . Question: Complete an MO energy diagram and determine the bond order for the NO.
(Use the energy ordering o Complete an MO energy diagram and determine the bond order for the NO. (Use the energy ordering of N2.) Drag the appropriate labels to their %(1). Complete the MO energy diagram for the N2+ ion by dragging the electrons Electron with spin up., ↑, ↑↓, ↓ in the figure given below.

Apply molecular orbital theory to determine the bond order of Ne2. 0.
OTHER SETS BY THIS CREATOR. 10 terms.
SOCIAL RESEARCH CH . Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the whole schematron.org this theory, each molecule has a set of molecular orbitals. Objectives: Practice energy diagrams for molecular orbital theory.The Hydrogen Molecule Ion H2+Molecular orbital diagram – Wikipedia