Lewis structure example.
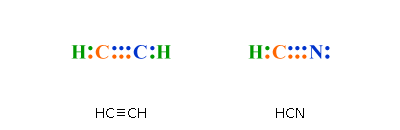
Draw the Lewis Structure of HCN. 1. Draw the skeletal structure showing how the atoms are connected using single bonds.

Usually try. Jul 4, Learn to draw the Lewis structure of HCN & understand molecular geometry, This is how Lewis dot structure of Hydrogen Cyanide goes!.
Nov 12, Draw a skeleton structure. Put the least electronegative atom C in the middle with H and Cl on either side. H-C-N.
Step 2. Count the valence. Draw a skeleton structure.
Sigma and Pi Bonds: Hybridization Explained!
Put the least electronegative atom C in the middle with H and Cl on either side. H-C-N. Step 2.
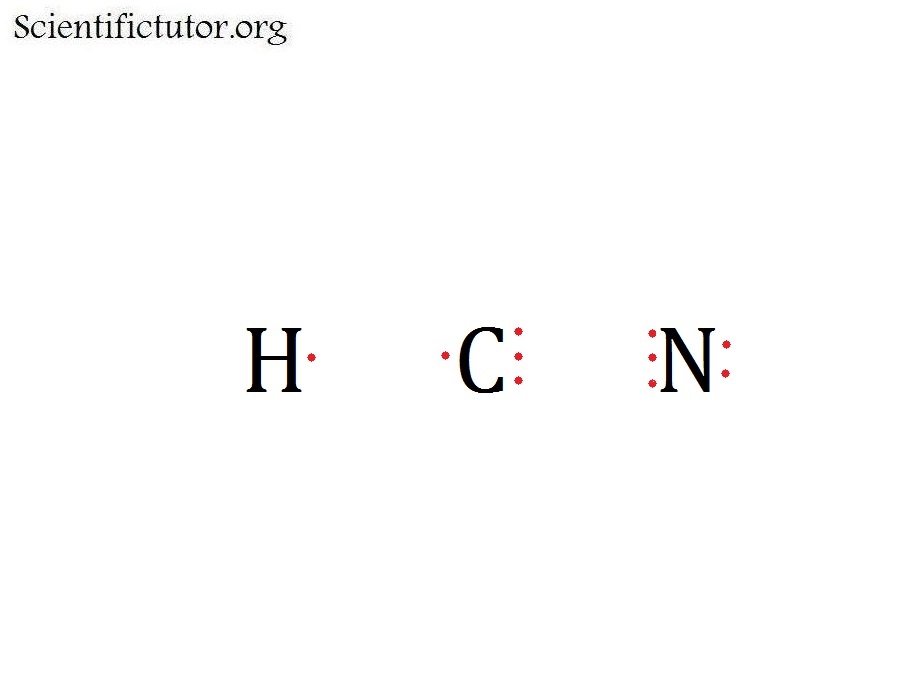
Count the valence. Lone pairs are not shown in a structural diagram C2H6 H H H C C H H H Electron Dot Diagrams HCN Electron Dot Diagrams HCN Electron Dot Diagrams HCN.Feb 23, · Best Answer: well in HCN carbon and nitrogen share three electrons(one is a pie bond if i am not much mistaken), thus nitrogen attains teh stable octet configuration but carbon is still short of one electron so it shares one electron with hydrogen thus enabling carbon to Status: Resolved. The difference between the Lewis dot structure and the structuralformula is that the formula only shows the bonds that have formedwhereas the dot structure shows all the valen ce electrons,including lone pairs, in that molecule.

Lewis dot structure of HCN. Alternatively a dot method can be used to draw the lewis structure of BF 3. Calculate the total valence electrons in BF 3 molecule.

H:1 C:4 N Total= Put carbon in the center and hydrogen and oxygen on the sides. Feb 07, · Electron Dot Structure for ethene, C2H4. The key to understanding how to distribute the valence electrons is to recognize the need for a double bond between the two carbon atoms.
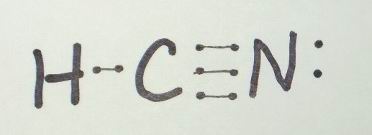
Transcript: For the HCN Lewis structure we have one valence electron for Hydrogen, we have four for Carbon, and we have five for Nitrogen, for a total of ten valence electrons for the HCN Lewis structure. We’ll put the Carbon in the center, because it’s less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures.HCN Lewis Structure [w/ free video guide]Hydrogen cyanide – Wikipedia