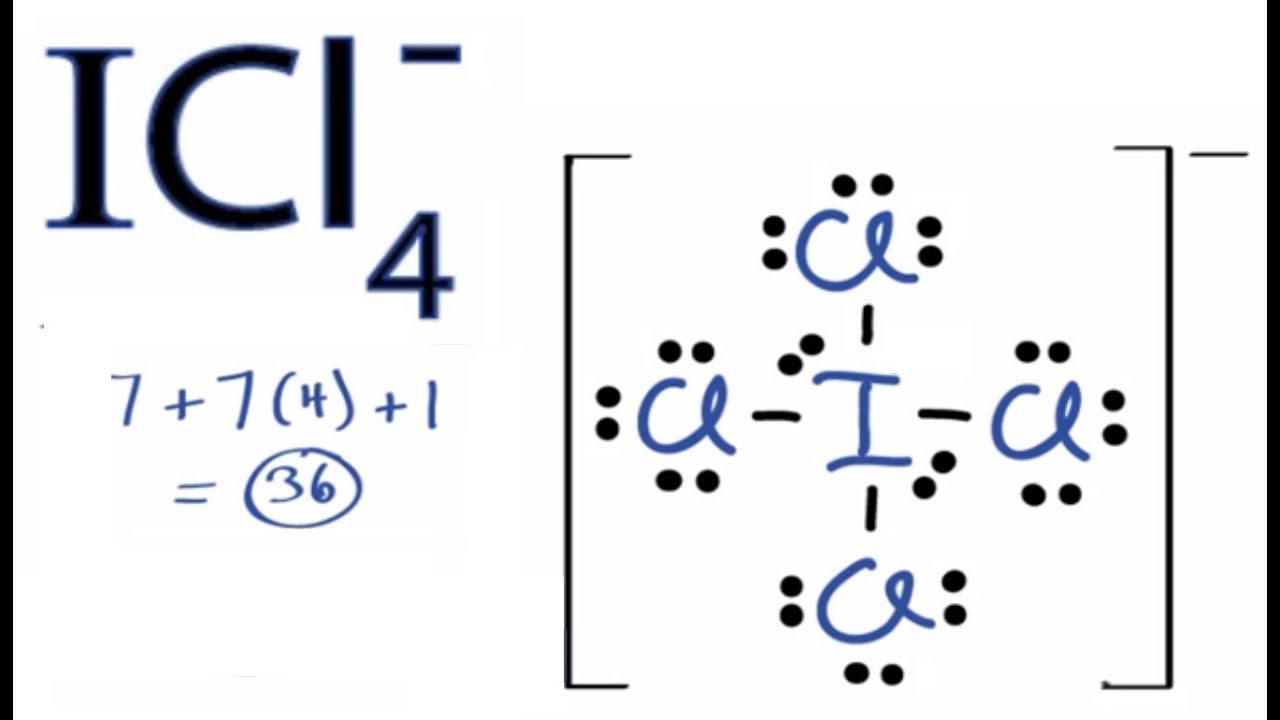
Comprehensive information for the element Iodine – I is provided by this page including scores of properties, Atomic Structure of Iodine Electron Dot Model . Lewis dot structures help predict molecular geometry. This example Total valence electrons to be “happy” = 1 iodine (8) + 3 chlorine (3 x 8).
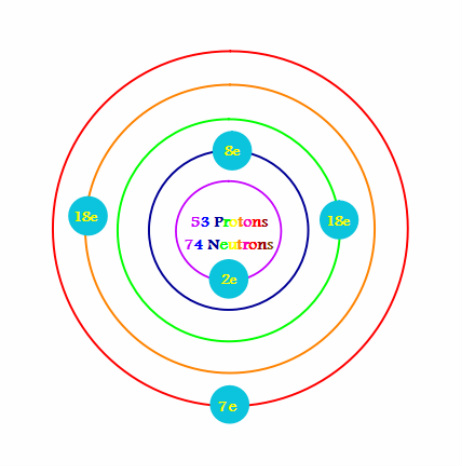
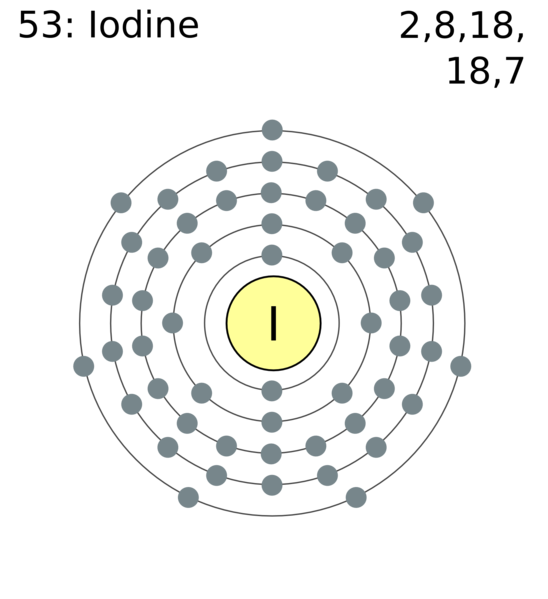
The iodine atom from group VII has 7 valence electrons. Hence in order to achieve stable octet configuration, two iodine atoms share a pair of valence electrons to form an I-I single covalent bond. How can you determine the Lewis dot structure of iodine heptafluoride?.
iodine has 7 valence electrons. So you draw seven dots, 2 on each side of the letter I.
and on one side you just put one dot, I think its best to put. The left diagram shows a Lewis dot structure of sodium with.
ASK A BRAND
Likewise, they can be used .. The left shows an iodine atom with one lone pair.
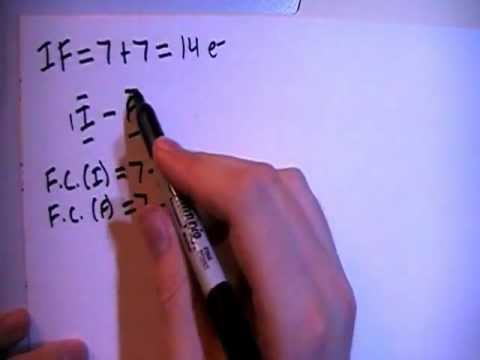
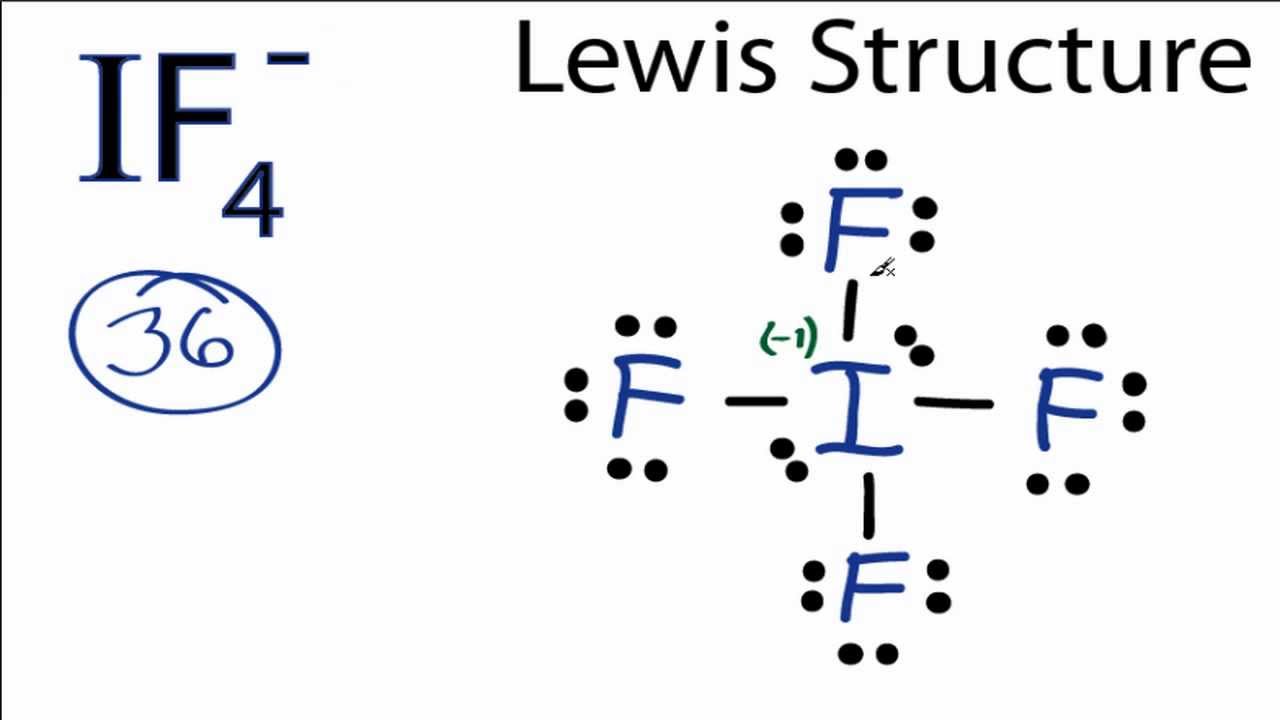
When we write the .Iodine having valence electrons in the 4th energy level, will also have access to the 4d sublevel, thus allowing for more than 8 electrons. IF 3 is dsp 3 hybridized and contains 2 lone pairs and 3 bonding pairs of valence electrons around the Iodine.
The VSEPR predicts the T-shape. Aug 05, · plz with a pic or if u cnt wit/o a pic jus tell me how it looks schematron.org: Resolved. Iodine pentafluoride is an interhalogen compound with chemical formula IF 5.
It is a fluoride of iodine. It is a colorless or yellow liquid with a density of g cm −3. Transcript: This is the ICl Lewis structure: Iodine Chloride.
For Iodine we have 7 valence electrons, and 7 for the Chlorine; total of 14 valence electrons for the ICl Lewis structure. We’ll put the Iodine here, and the Chlorine right next to it. The iodine atom from group VII has 7 valence electrons.

Hence in order to achieve stable octet configuration, two iodine atoms share a pair of valence electrons to form an I-I single covalent bond.What should the electron dot diagram for iodine look like? | SocraticLewis Dot of Iodine Trifluoride IF3