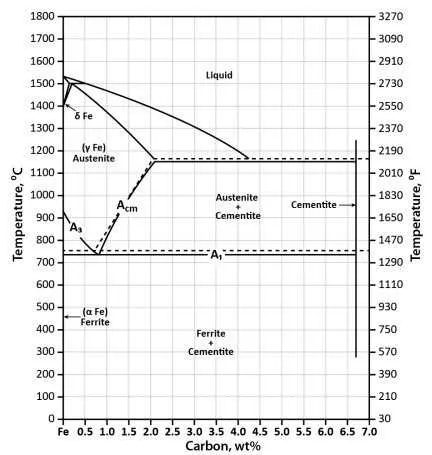
To accurately determine the properties of steel alloys, one must understand the phase diagram that governs the equilibrium of elements within the alloy. This chart provides critical information regarding the melting points, solubility limits, and phase transitions that occur at various compositions and temperatures. A thorough grasp of this system is essential for optimizing processes like welding, heat treatment, and casting.
When working with steel, the main focus should be on the solid-liquid phase transitions, which dictate the behavior during cooling or heating. In particular, the boundary lines in the chart, such as the eutectic point, indicate key temperatures where the alloy’s composition transforms from a single phase to a mixture of phases.
Professionals use this knowledge to predict mechanical properties like hardness, strength, and ductility. The regions of the chart show where certain phases, such as austenite or pearlite, are stable, helping metallurgists fine-tune the heat treatments to achieve the desired material properties for specific applications.
By carefully analyzing these boundaries and transitions, one can better control alloy behavior, ensuring that materials meet the stringent demands of high-performance industries such as automotive manufacturing or aerospace engineering.
Understanding the Phase Diagram of Fe-C Alloy
When analyzing the phase transitions of a ferrous alloy, focus on the eutectic composition and critical temperatures: the eutectoid transformation at 0.8% weight of the solute at 727°C, where austenite changes to pearlite. Below the solidus line, the material is completely solid, while above the liquidus, it is fully molten.
The upper critical temperature, known as the A3 point, defines the boundary for full austenitization, usually occurring at temperatures around 1394°C for pure iron. Below this point, ferrite and cementite phases form, with the solubility of the solute in ferrite decreasing with temperature. Understanding these transitions is key for controlling material properties in metallurgy.
Key to this alloy system is the interplay between microstructure, strength, and hardness. For instance, quenching from high temperatures can trap austenite and increase hardness, leading to martensite formation, which is crucial in producing high-strength steels.
For effective alloy design, it’s essential to utilize the boundaries of the phase fields to optimize heat treatment processes, controlling microstructures like pearlite, bainite, or martensite, depending on the intended mechanical properties.
Understanding the Phase Boundaries in the Iron-Carbon Diagram
The phase boundaries in the binary phase representation of iron and its alloying element are crucial for understanding the material’s behavior under varying temperature and composition conditions. These lines separate different solid and liquid states, and they define the formation of phases like ferrite, austenite, cementite, and liquid phases. Knowing these boundaries helps in controlling microstructure and mechanical properties.
- Liquidus Line: This line marks the transition from solid to liquid as the temperature rises. The liquidus is crucial for casting and welding processes, where the material transitions from solid to molten.
- Solidus Line: Below this line, the alloy is completely solid. Understanding the solidus is important for processes like heat treatment, where the cooling rate determines the final microstructure.
- Eutectoid Point: This specific point represents the temperature and composition where austenite transforms into a mixture of ferrite and cementite. It is essential for determining the ideal cooling rates for creating pearlitic structures in steel.
- Eutectic Point: Occurs where liquid transforms directly into two solid phases. In steel metallurgy, this is important for the formation of pearlite from liquid phases during solidification.
To achieve desired material properties, understanding the boundaries helps in tailoring heat treatment processes like annealing, quenching, and tempering. Fine-tuning compositions based on these limits ensures the desired mechanical and thermal properties in finished products.
Practical Applications of the Iron-Carbon Diagram in Steel Production
Understanding the phase relationships in metal alloys is crucial for producing specific material properties. The phase chart is essential for controlling solidification and transformation processes in various steel grades. It helps in predicting the temperature range for critical transformations such as austenitizing, hardening, or tempering, ensuring the desired mechanical properties of the final product.
During heat treatment, accurate control of cooling rates is vital. Using the phase chart, engineers can determine whether the cooling process will lead to the formation of pearlite, martensite, or bainite structures, which directly affect hardness, strength, and ductility. For instance, rapid cooling may result in martensitic structures, while slower cooling can lead to a more ductile pearlitic structure.
In alloy development, knowing the specific points where phase changes occur allows for fine-tuning the material composition. By adjusting the content of other elements like manganese, chromium, or silicon, producers can tailor the properties of the metal for specific applications such as tools, automotive parts, or construction materials.
Quality control in large-scale production benefits from the ability to predict microstructural changes. Engineers use the phase chart to verify that products meet required specifications, adjusting heat treatment cycles to optimize performance. The chart also assists in the detection of potential defects, such as undesired phases or improper cooling rates that may compromise product integrity.
Impact of Carbon Content on the Properties of Metal Alloys
Increasing the concentration of carbon in metallic compounds enhances their hardness and tensile strength. However, this comes at the cost of reduced ductility and toughness. Alloys with a high percentage of carbon are harder but more brittle, making them suitable for tools and machinery parts where wear resistance is a priority. For instance, compounds with over 2% of carbon generally exhibit significant hardness but lose their ability to deform without fracturing.
Metallurgists often manipulate the levels of this element to achieve the desired balance between hardness and flexibility. As the content rises, the material transitions through several phases, each affecting its performance characteristics. For example, a material with around 0.8% of this element tends to have an optimal balance, exhibiting both strength and malleability. Beyond 1%, the increase in hardness becomes more pronounced, but the material becomes less capable of absorbing impact without cracking.
Alloys containing higher percentages are primarily used in applications requiring wear resistance, such as cutting tools, whereas those with lower levels are preferable in structural components that need to withstand bending or deformation under stress. Heat treatment processes, such as quenching and tempering, are essential for adjusting the properties of these metals, allowing for further fine-tuning of their mechanical properties.