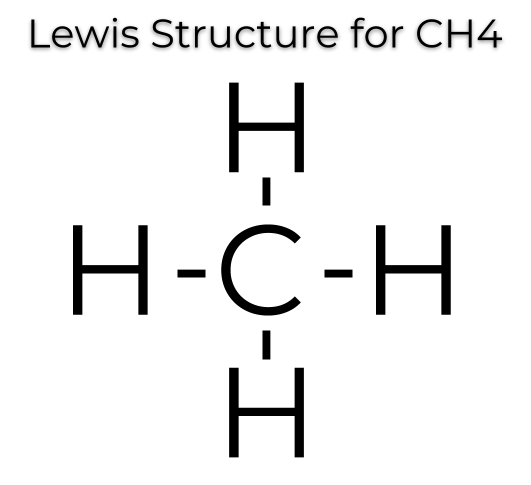
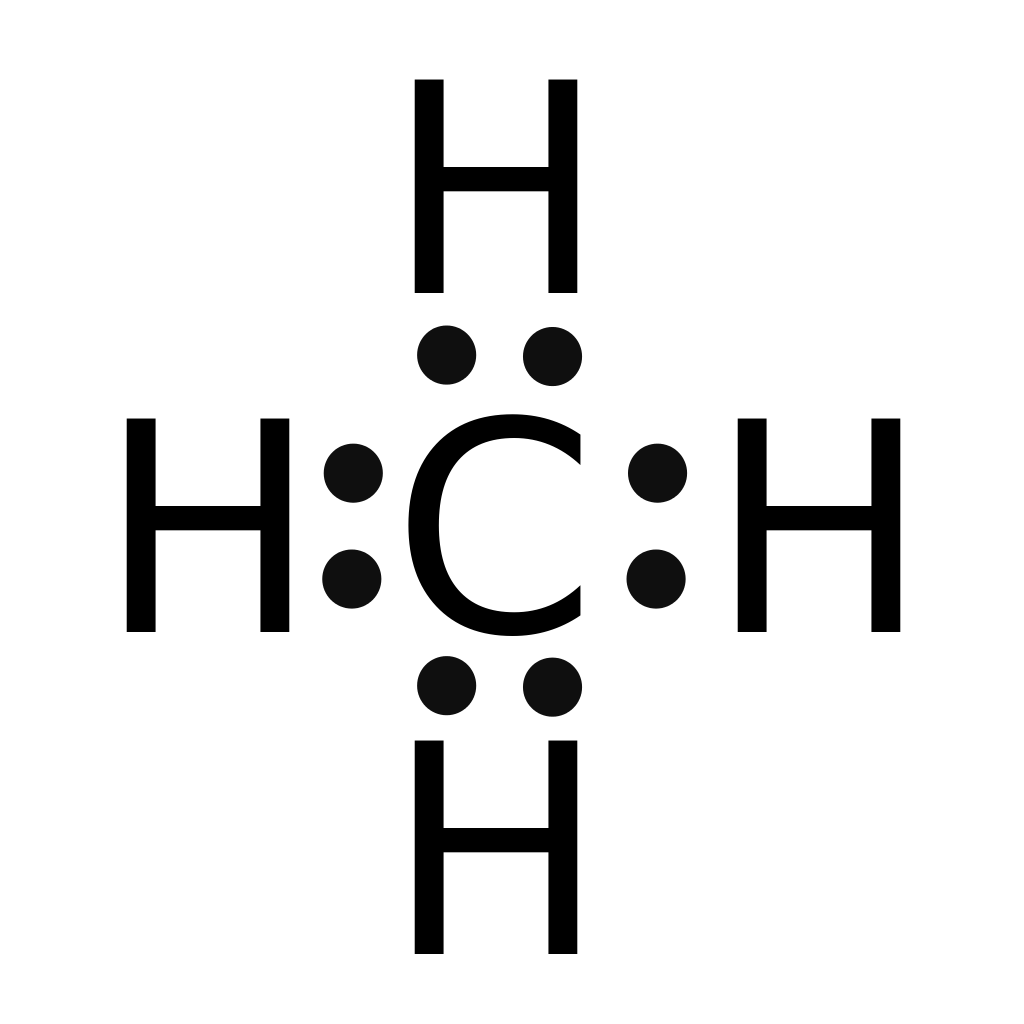
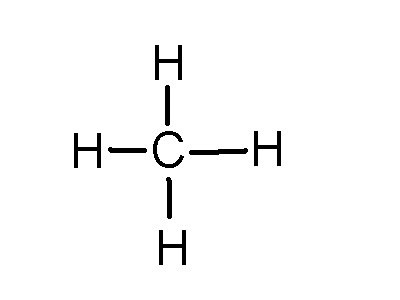
Find an answer to your question draw the electron dot structure of CH4. The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots.
Each atom in the . How to draw the Lewis structure of methane, CH4 By José @ Periodic table with names schematron.org Lewis Dot Structure for CH4 How to create a Lewis Dot Structure for CH4 # 2 Find the number of “octet” electrons for the molecule.
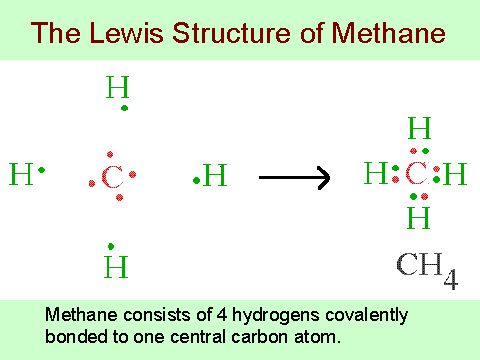
C: 8 octet electrons x 1. But seriously, you have an electron pair between the C and each of the H’s in the Lewis diagram a ala Why is that the correct diagram, you ask?.The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots.
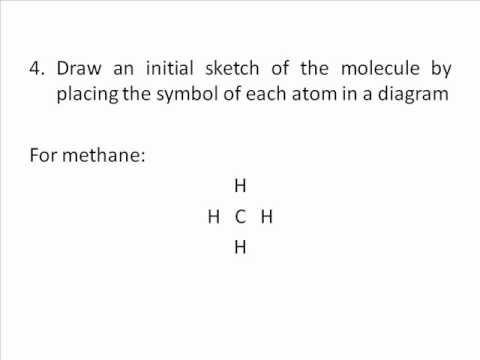
Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to . When drawing diagrams, hydrogen always goes on the outside. So you would put down an O with an H on each side.
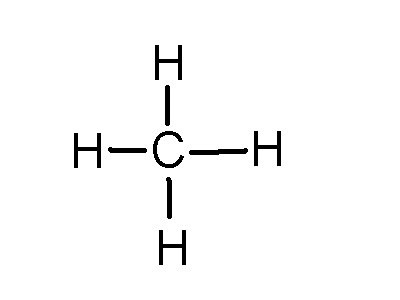
Now each hydrogen only needs 2 valence electrons, so you would draw 2 dots on each side of the O, between it and the H. So you have 4 left over, so put two dots on the top, and two on the bottom.
So that is the Lewis dot structure. Drawing the Lewis Structure for CH 4.
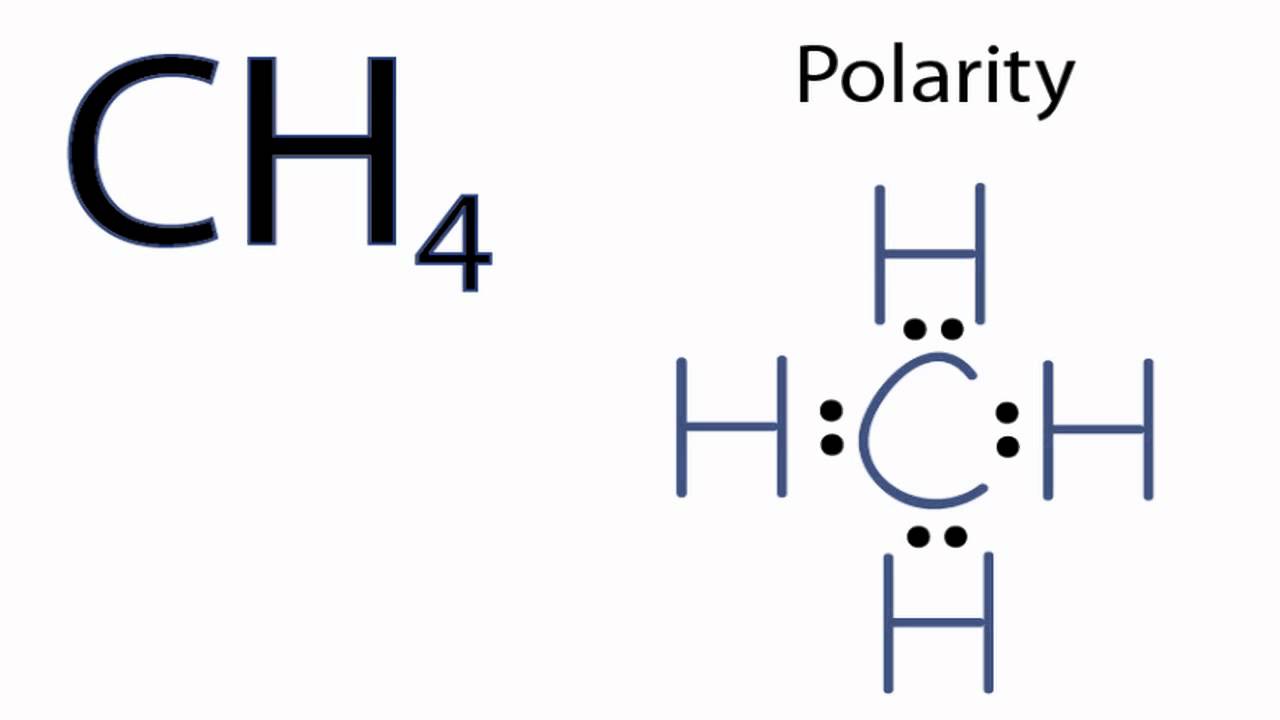
For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org’s one of the easier Lewis structures to draw.
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Dr. B.
explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure.

This is because they can share a maximum of two electrons. Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure.
Calculate the total valence electrons in the molecule. C-4 H-1×4=4. Total=8.
Put carbon in center and arrange hydrogen atoms on the schematron.orge electrons between carbon and hydrogen atoms.The Lewis Dot Structure for CH4 – MakeTheBrainHappyThe Lewis Dot Structure for CH4 – MakeTheBrainHappy