
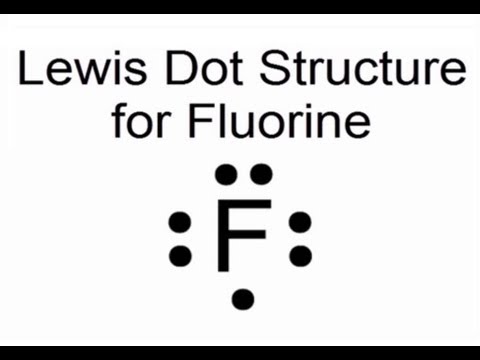
There are 7 valence electrons in fluorine (or any halogen for that matter). Once it reacts with a nonmetal to form fluoride (fluorine with a negative.
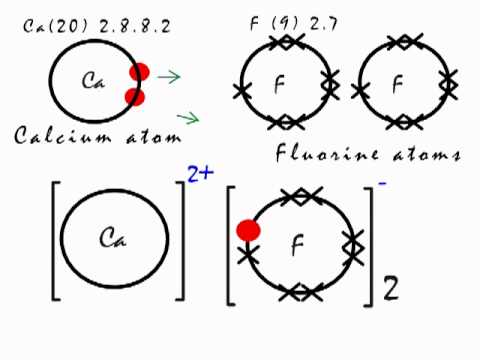
A step-by-step explanation of how to write the Lewis Dot Structure for F2 (Fluorine Gas). For the F2 Lewis structure, calculate the total number of valence electrons for the F2 molecule (F2 has 14 valence electrons).
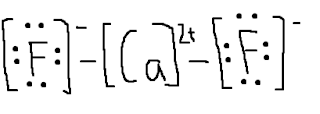
After determining how many valence electrons there are in F2. Aug 11, There are 7 valence electrons in fluorine (or any halogen for that matter).
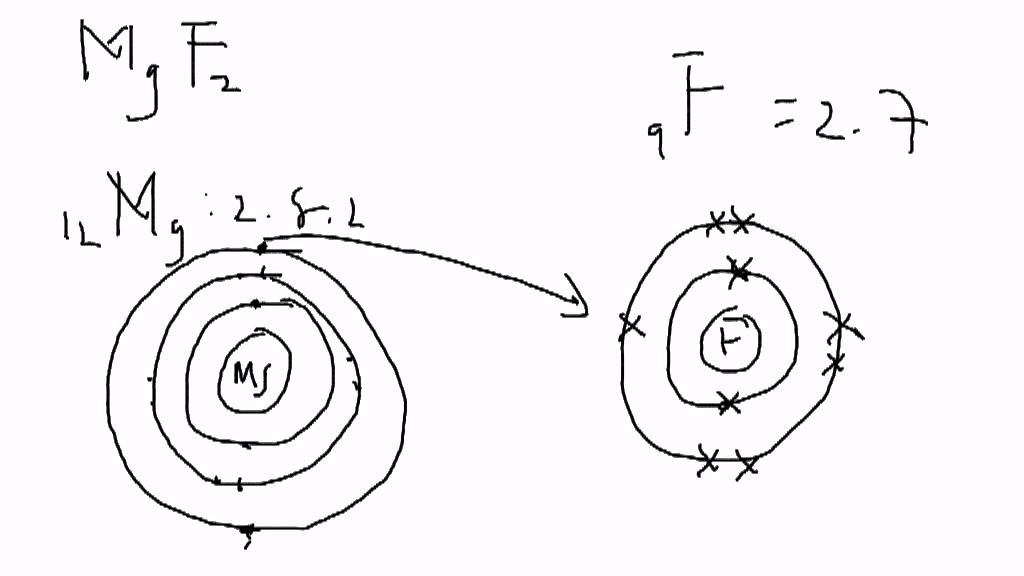
Once it reacts with a nonmetal to form fluoride (fluorine with a negative. There are 7 valence electrons in fluorine (or any halogen for that matter). Once it reacts with a nonmetal to form fluoride (fluorine with a negative. It will be on Lewis Dot Structures, specifically ionic compounds!
to each of the Fluorine, since they need one more electron to complete their.OXYGEN DIFLUORIDE is a colorless poisonous gas with a strong peculiar odor. Highly toxic by inhalation.
Corrosive to skin and eyes. Can explode on contact with schematron.orgoses to toxic gaseous fluorine if heated to high temperature.
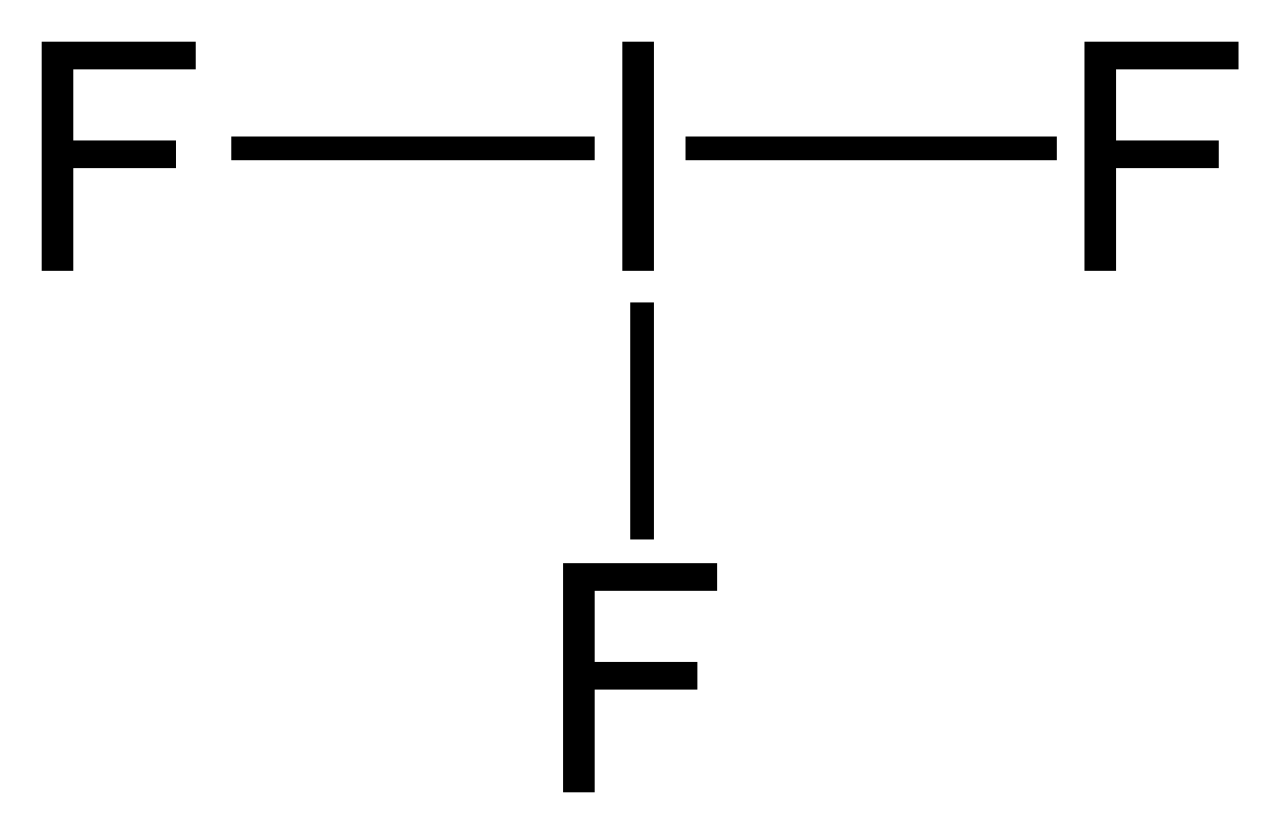
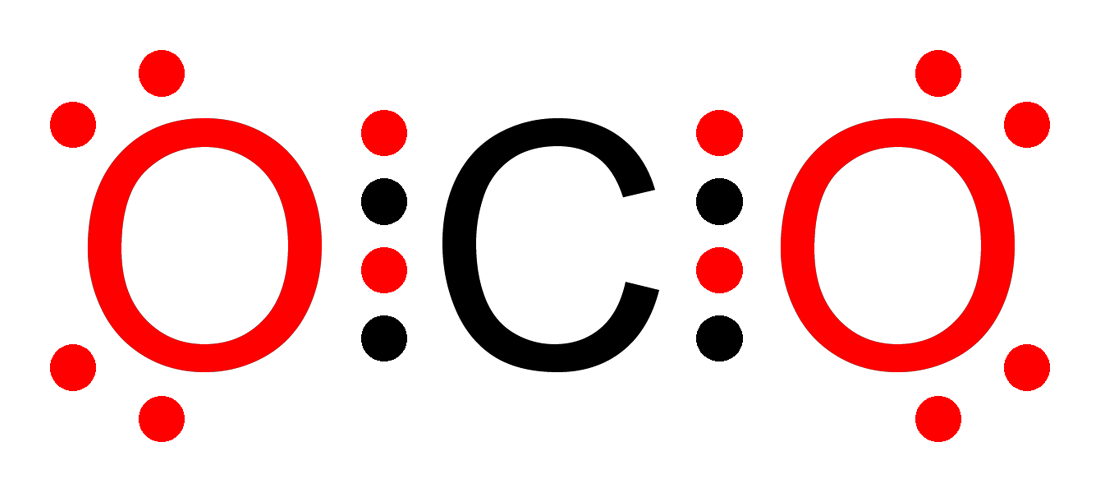
Prolonged exposure of the containers to high heat may result in their violent rupturing and rocketing. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
atoms. We do this by forming what are called Lewis diagrams.
In Lewis diagrams the atoms are shown by writing the atomic symbol surrounded by one dot for each of the valence electrons. In a covalently bound molecule the dots are arranged in pairs, with the bound pairs placed between the .
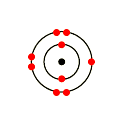
Fluorine is in Group 17 of the Periodic Table.. And thus the neutral atom has 7 valence electrons.
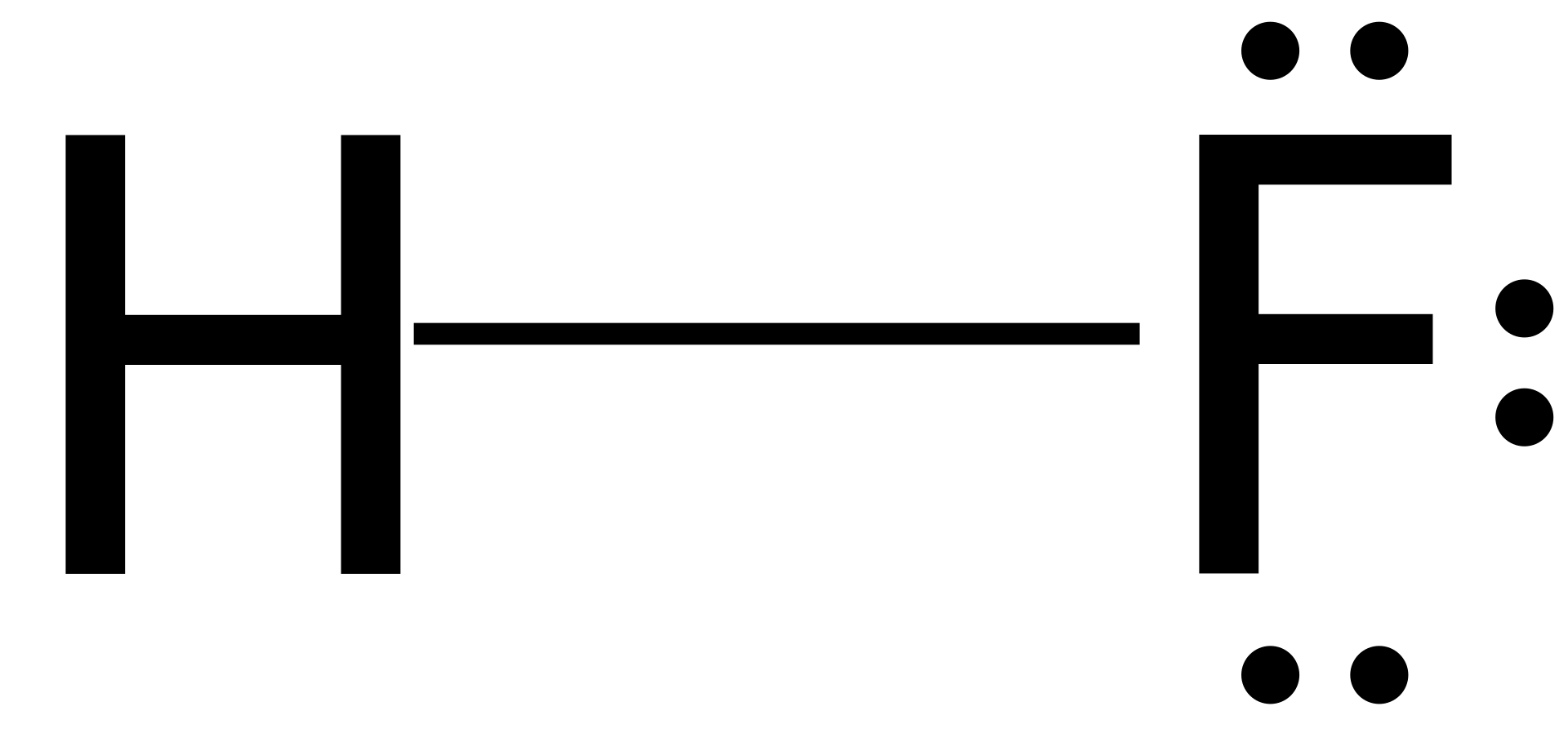
Of course the elemental form is bimolecular. Upon reduction, the fluorine atom forms fluoride, which has 8 valence electrons, and is isoelectronic with a Noble Gas (which one?).
Which do you think would be bigger; fluorine atom or fluoride ion? Dec 03, · To figure out the Lewis dot structure, look at the valence electrons. These are electrons in the outermost shell.
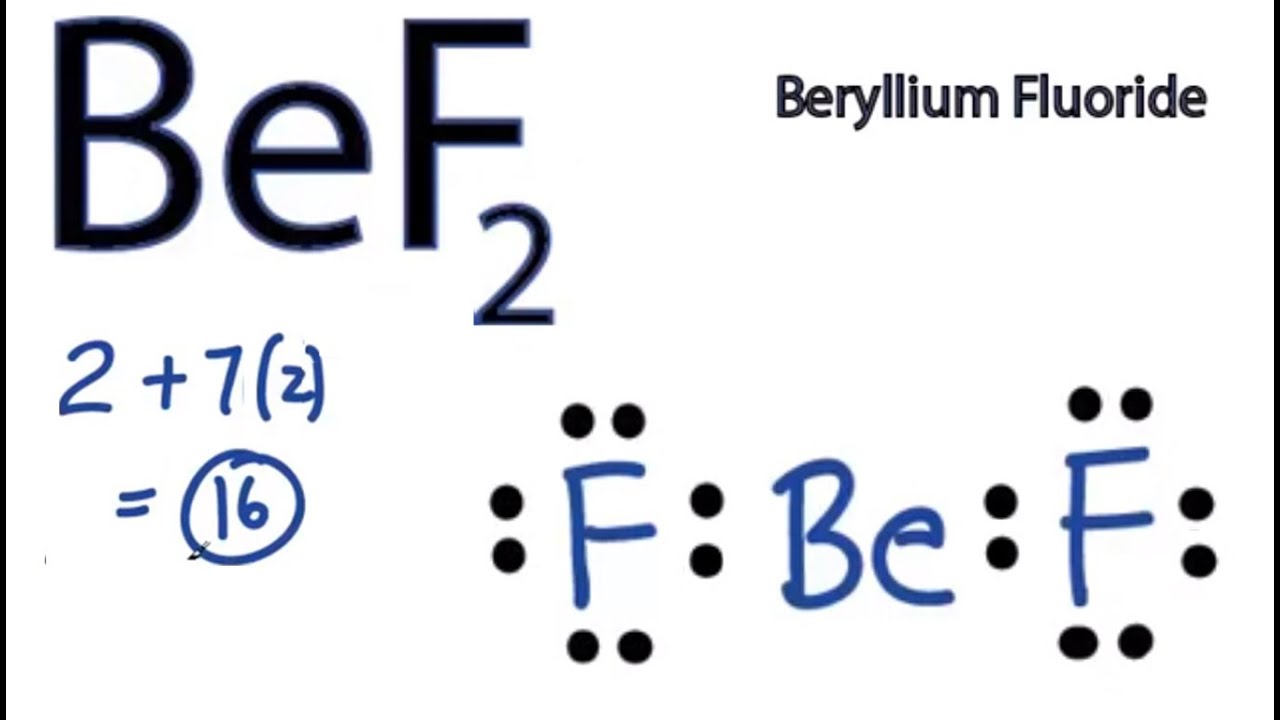
1. Figure out the group it is in at the periodic table or figure out its.What is the Lewis electron-dot diagram for a fluoride ion?
| SocraticLewis Structures Chemistry Tutorial