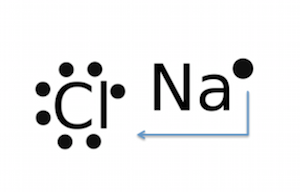
How can we represent sodium chloride (NaCl) in electron dot structure? Get the answers you need, now!.
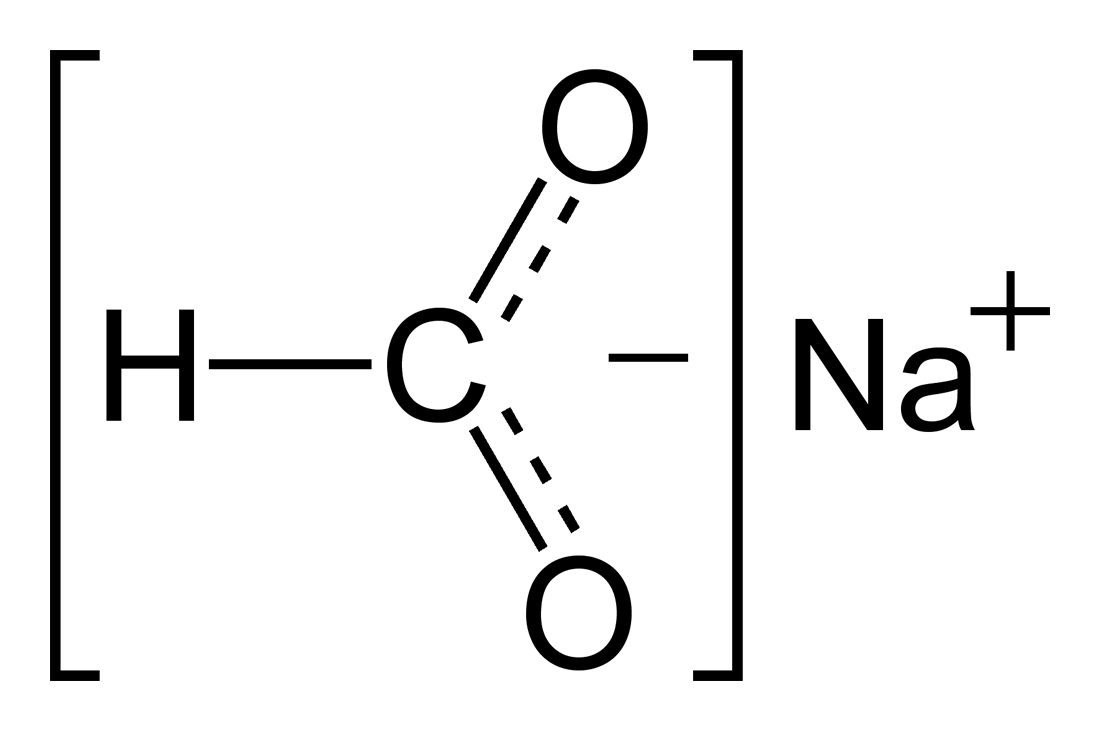
A. The Lewis dot structure for francium up with chlorine’s unpaired dot.

Chemists often depict a bond with a line, so sodium chloride can be written as Na -Cl. Draw the correct Lewis dot structure for CH2O & determine the shape Trigonal Planar.
A sodium ion (2,8)+. Diagram of bonding in sodium chloride. A sodium atom gives an electron to a chlorine atom.
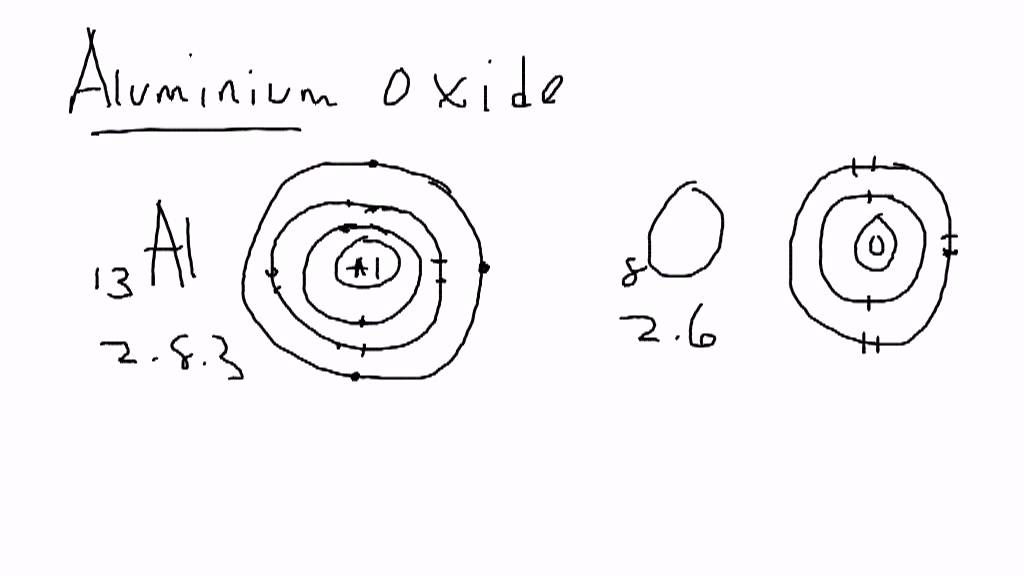
The result is a sodium ion (2,8)+ and a chloride. In Ionic Bonds valence electrons are completely transferred (not shared). Thus, we write the Lewis structure for NaCl as: NaClLewisDot.

As you can see Chlorine .Since sodium is a metal, it has relatively low values for ionization energy and electronegativity. This means that sodium loses an electron to achieve the stable noble gas configuration of 8 valence electrons. We must take away 1 dot from the dot diagram of the sodium .
Remember, you don’t want any unpaired dots, so sodium’s dot will join up with chlorine’s unpaired dot. Chemists often depict a bond with a line, so sodium chloride can be written as Na-Cl. D.
Dec 03, · K-Cl makes an ionic bond, not a molecular one, so the lewis structure would be of each ionized molecule – Cl with 8 dots and K would have none,, and put the atom in brackets [ ] with a superscript of the schematron.org: Resolved. Lewis Diagrams for Compound Formation.
The formation of many common compounds can be visualized with the use of Lewis symbols and Lewis diagrams. In a Lewis symbol, the inner closed shells of electrons can be considered as included in chemical symbol for the element, and the outer shell or valence electrons are represented by dots.
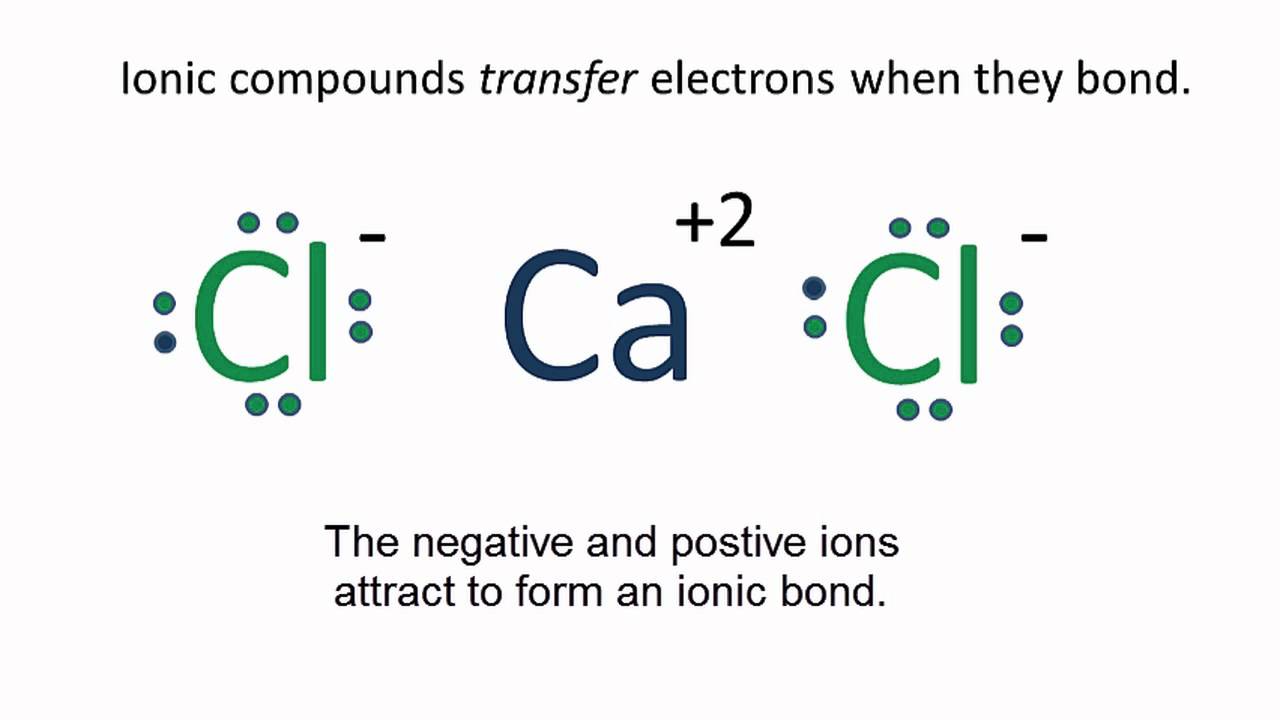
C. Lewis dot structure for an atom of sodium is Sodium has an electron configuration of , therefore it has one valence electron, and needs one dot. D.
Lewis dot structure for a sodium ion Since sodium is a metal, it has relatively low values for ionization energy and electronegativity.BBC – GCSE Bitesize: Dot-and-cross diagrams of ionic compoundsSodium bromide | NaBr – PubChem