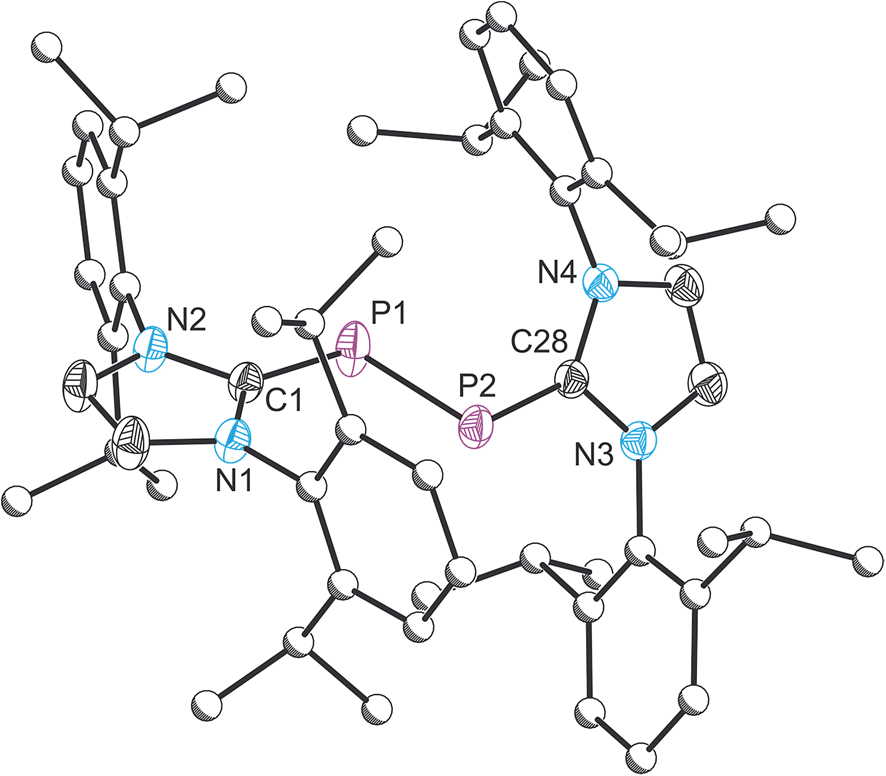
Valence MO diagram (not all tie-lines drawn): nb. H(A). 2pBe .
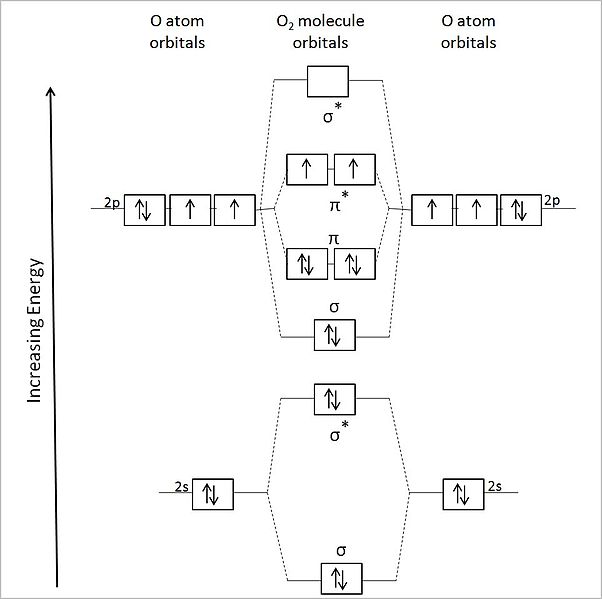
Ozone. Ozone is planar, so there will be one p orbital from each oxygen atom.
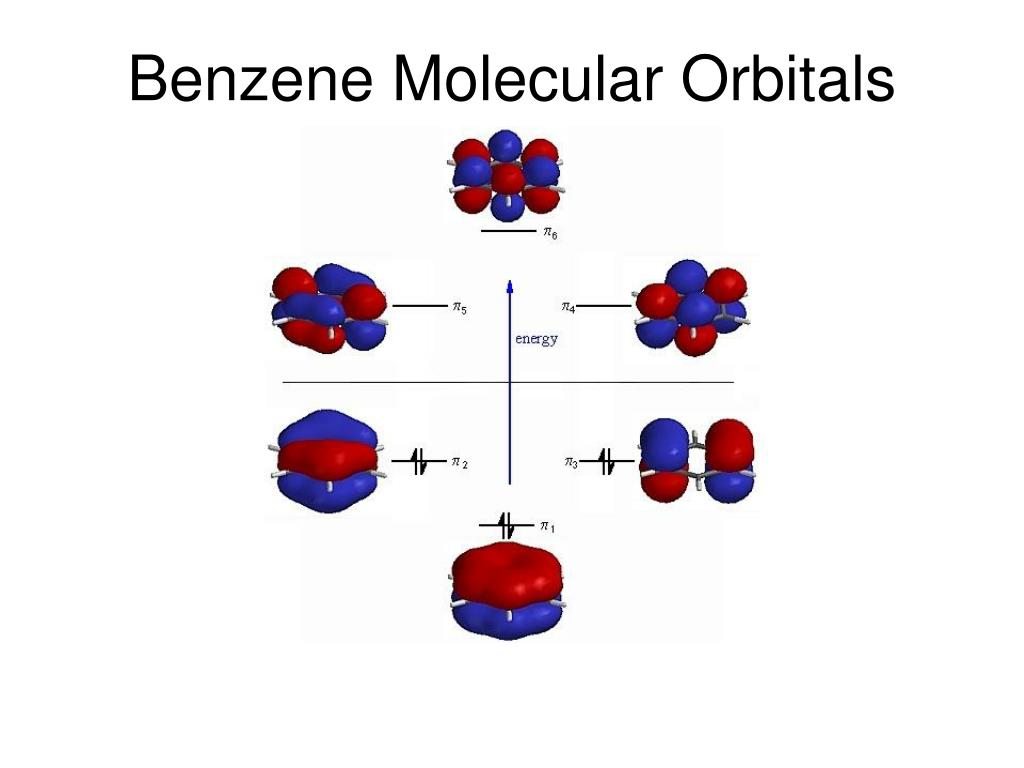
All molecular orbitals except the highest would be occupied by molecular orbitals in the diagram .. and 10 in the ozone diagram in the Problem answer.
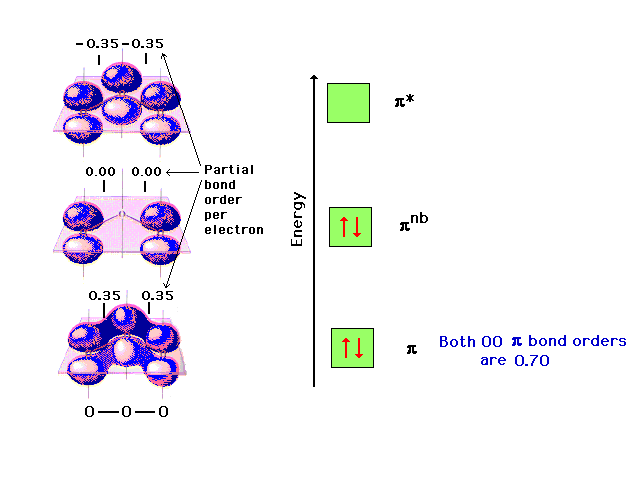
The ozone molecule’s Lewis structure shows that even the preferred structure The pi molecular orbital energy diagram for ozone into which are distributed four . Our MO treatment of ozone is entirely analogous to the 4-electron H3- anion. We map that solution.
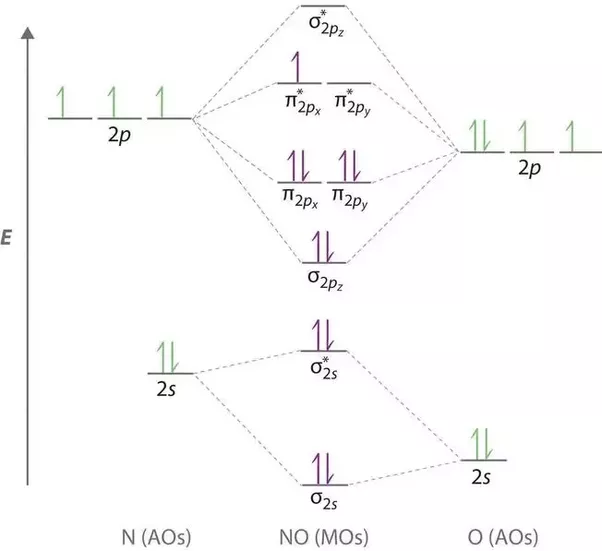
Figure Molecular Orbital Energy-Level Diagram for \(\pi\) Each oxygen atom in ozone has 6 valence electrons, so O 3 has a total of Jan 10, · Alright, let’s be real. Nobody understands molecular orbitals when they first take chemistry. You just pretend you do, and then in your next course you learn them a little better.
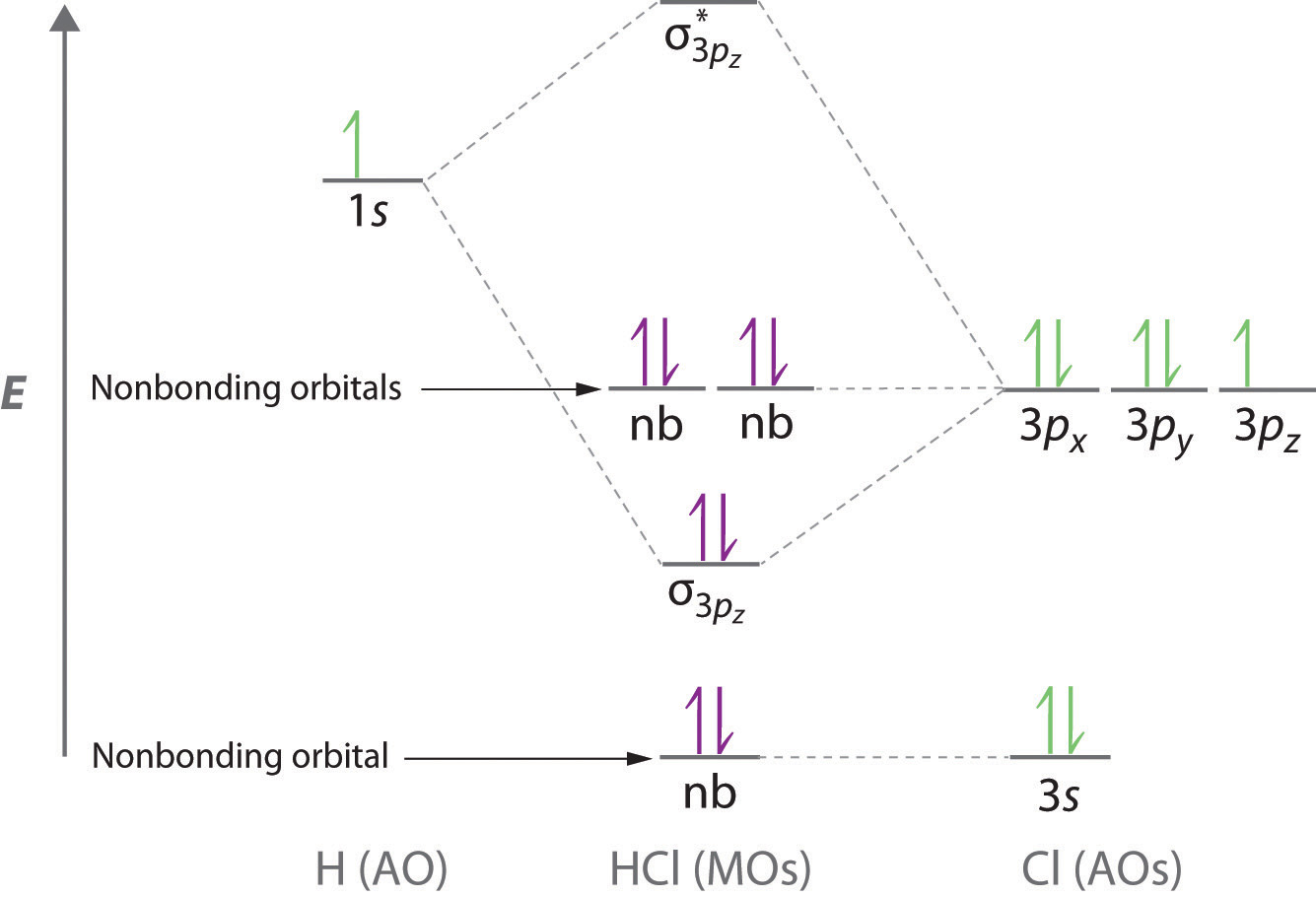
Compare the bond order in H 2 + and H 2 using the molecular orbital energy diagram for H 2. The bond dissociation energy of the H 2 molecule is kJ/mol.
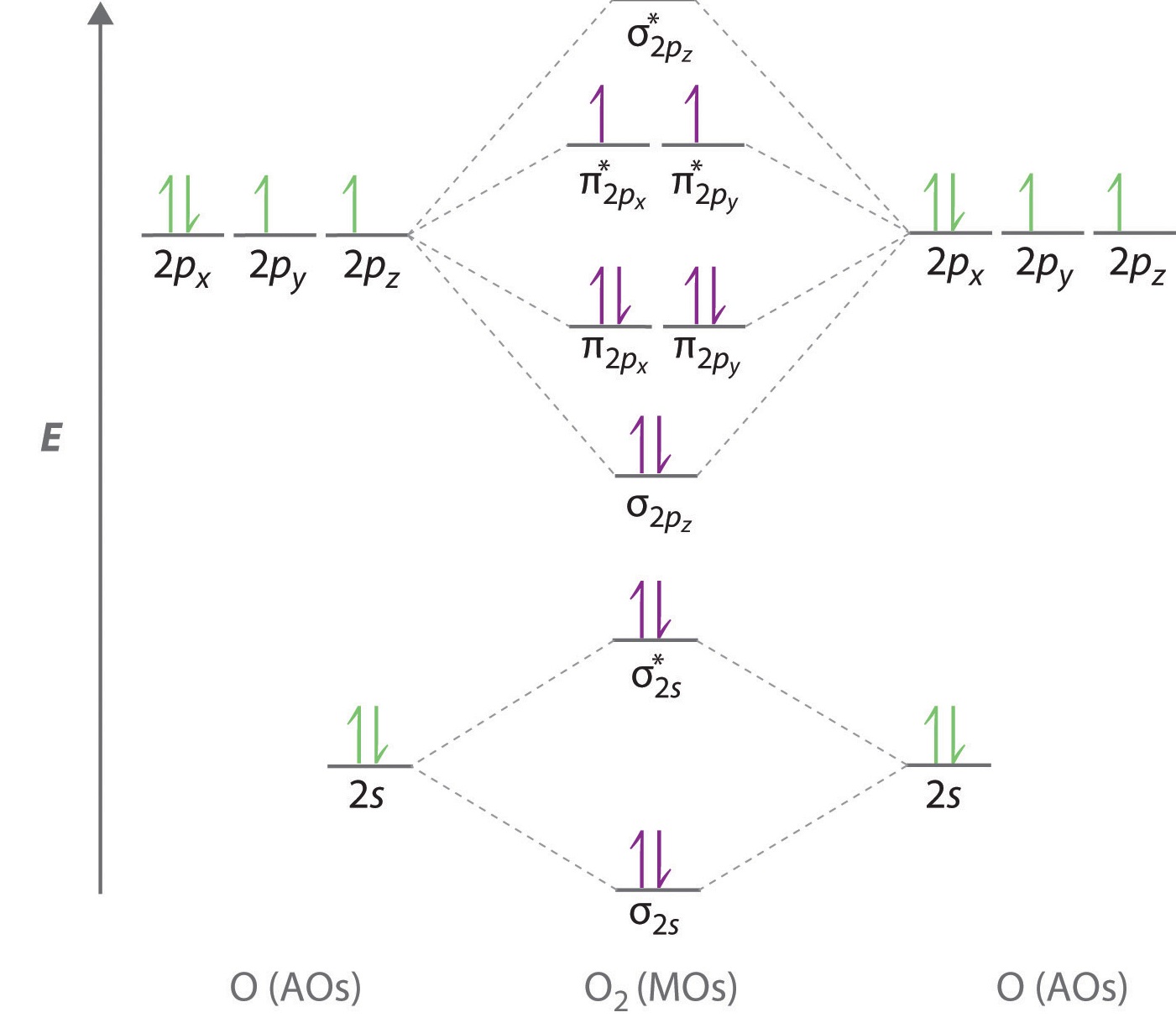
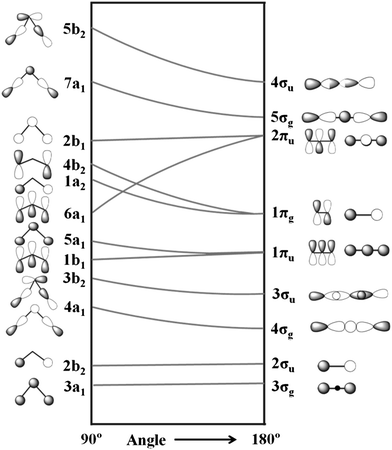
Explain why . Extended Pi Bonding In symmetry-based molecular orbital diagrams for the multiatom molecules water, ozone, and methane, we’ll combine group orbitals with the valence orbitals of the central atom.
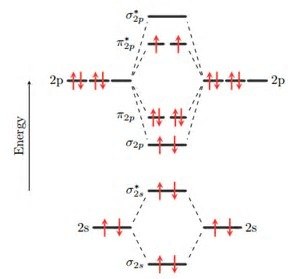
The group orbitals are linear combinations of atomic orbitals from all the atoms bonded to the central atom. Molecular Orbitals.
Delocalized Bonding: Conjugation in Ozone. Ozone is a fairly simple molecule, with only three atoms.
However, in order to focus on one aspect of ozone’s structure, we will use a hybrid approximation in order to simplify the picture. The Lewis structure of ozone is somewhat unsatisfactory.
Ozone is the unstable triatomic form of oxygen, O3. It is a powerful oxidant that is produced for various chemical and industrial uses. Its production is also catalyzed in the ATMOSPHERE by ULTRAVIOLET RAY irradiation of oxygen or other ozone precursors such as VOLATILE ORGANIC COMPOUNDS and NITROGEN OXIDES.Pi Bonds over 3 AtomsMolecular orbital diagram – Wikipedia