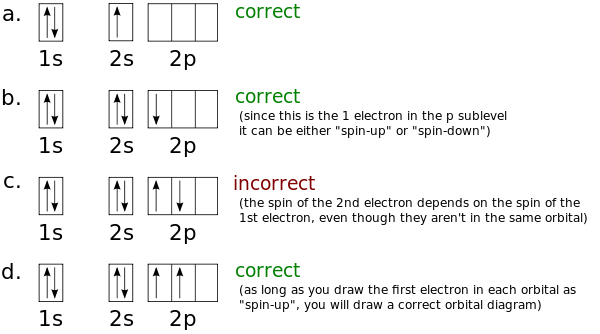
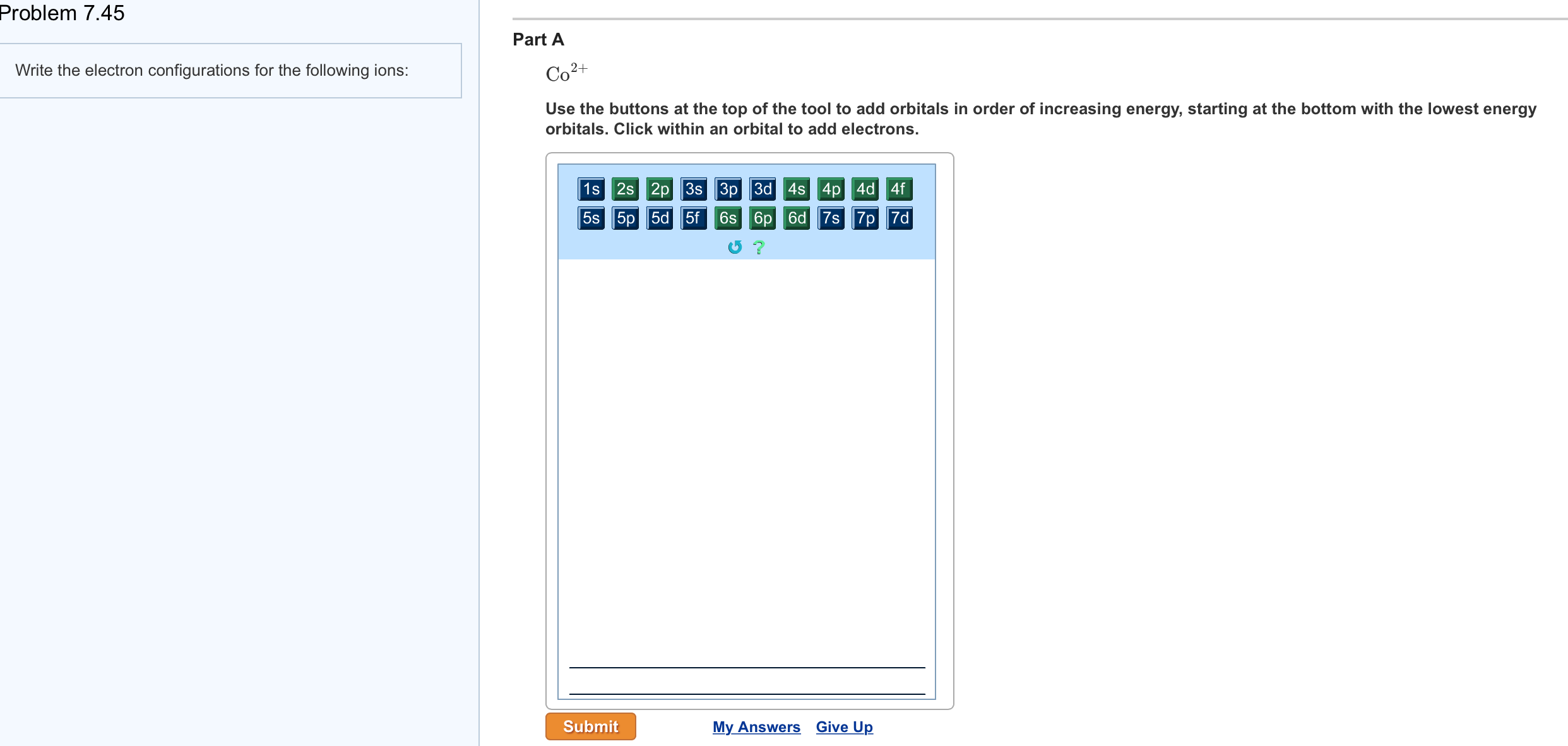
Part B. Draw the orbital diagram for the ion Co2+.
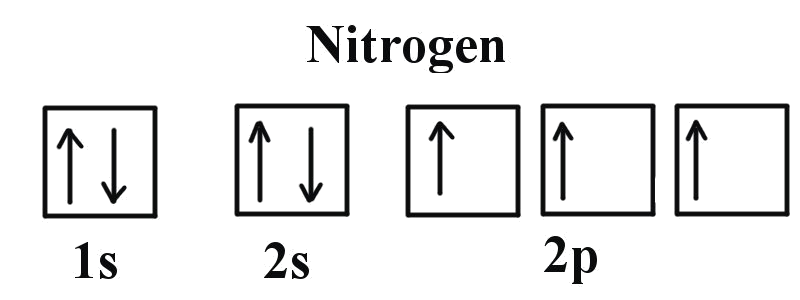
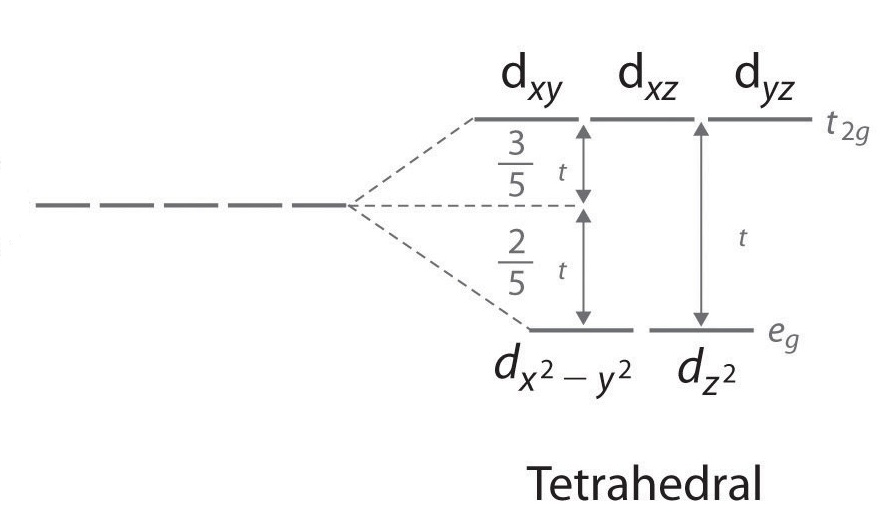
Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at. Ni2+ Draw the d-orbital splitting diagrams for the octahedral complex ions of each of the following. a.
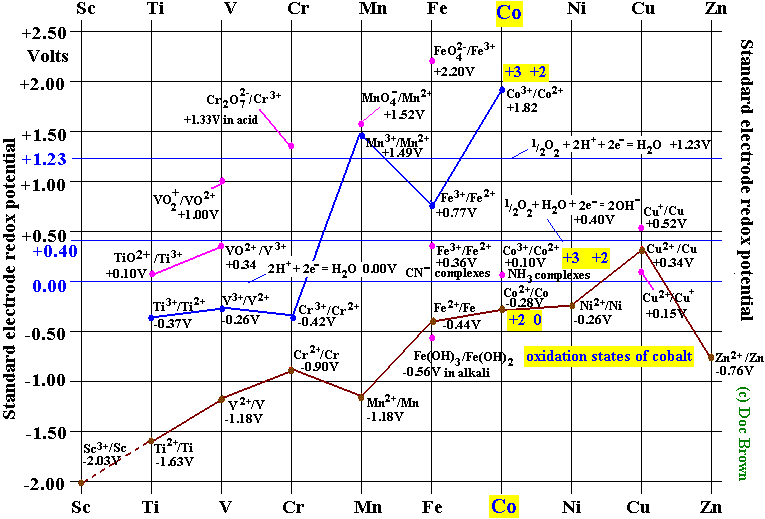
Zn2+ b. Co2+ (high and low spin) c. Ti3+ the FT ligand. Why isn’t it [Ar] 4s2 3d5 (considering the fact that half filled orbitals confer If you have a gas phase Co(2+) ion, it would likely reorganize such.
Watch the video solution for the question: Draw the orbital diagram for ion Co 2+.. .
Part B. Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the.Oct 05, · Also Cobalt orbital diagram example problem.
For more chemistry help videos and practice worksheets go to: http Example showing how to draw orbital diagrams. home / study / science / chemistry / chemistry questions and answers / Part A Write The Electron Configuration For Each Ion. Co2+ N3 Ca2+ Express Your Answers Question: Part A Write the 92%(13).
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals molecular orbital method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of molecular . Part B.
Draw the orbital diagram for the ion Co2+. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals.
Click within an orbital to add electrons. Part C.

Draw the orbital diagram for the ion N3−. Use the buttons at the top of the tool to add orbitals in order of increasing energy, starting at the bottom with the lowest energy orbitals%(8). The valence electron configuration of “O” is [“He”] 2s^2 2p^4.
To accommodate the two lone pairs and the bonding pair, it will also form three equivalent sp^2 hybrid orbitals. Two of the sp^2 orbitals contain lone pairs, while the remaining sp^2 orbital and the unhybridized p orbital have one electron each.How to draw the orbital diagram of Cobalt? | Yahoo AnswersDraw the orbital diagram for ion Co 2+ | Clutch Prep