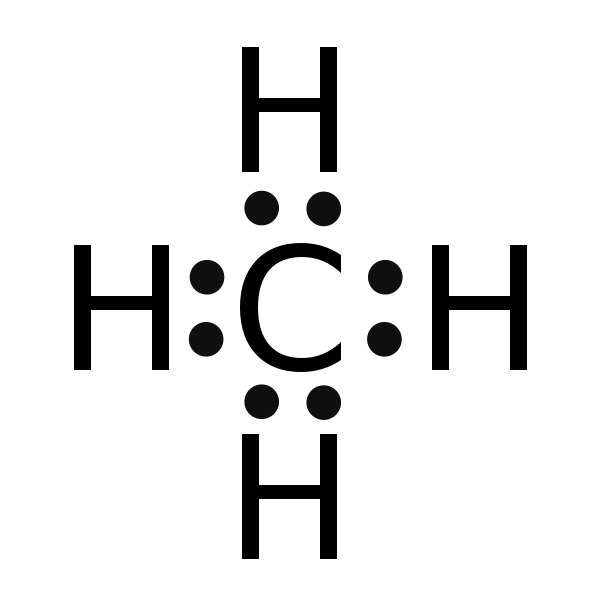
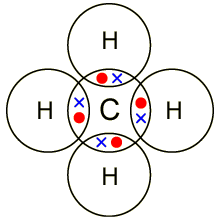
Well Carbon only has 4 valence electron, so it can bond at all four point.
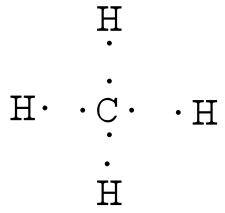
Hydrogen only has one valence electron and can only share one. Lewis symbols (also known as Lewis dot diagrams or electron dot diagrams) .
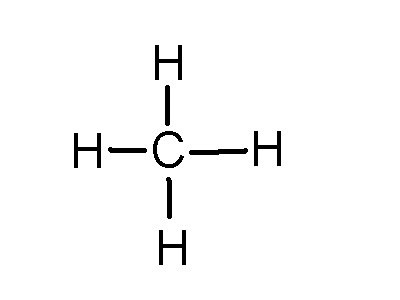
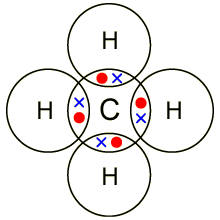
Lewis dot dragram for methane: Methane, with molecular formula CH4, is shown. It is important to remember that Lewis valence dot diagrams are models that Methane is the main component of natural gas, and its chemical formula is CH4. You will also need to be able to draw dot-and-cross diagrams representing the covalent Four pairs of electrons are shared in a methane molecule (CH4).
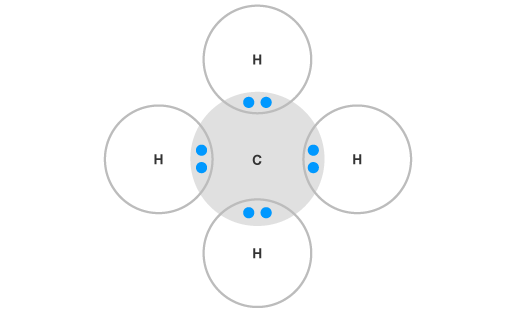
Electron Dot Structures – Helpful tools in thinking about bonding. Pictorial Electron dot structure – valence electrons are represented by dots placed around the chemical symbol.

Electrons are How about C and H? (methane – natural gas).Dr.
B. explains how to draw the Lewis dot structure for CH 4 (methane).
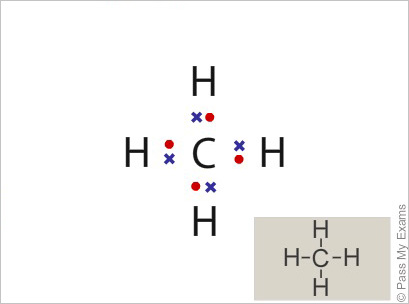
The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure.
This is because they can share a maximum of two electrons. Check the Formal Charges to make sure you have the best Lewis Structure.
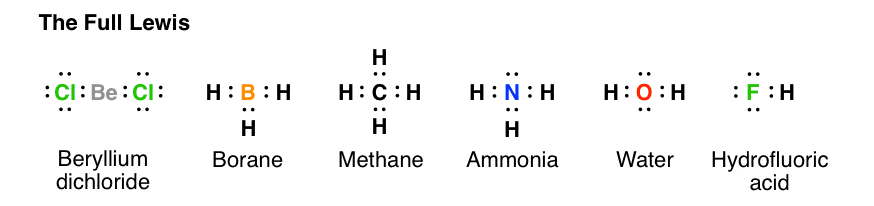
Explain How Examples: SO 4 2-, N 2 O, XeO 3; Notable Exceptions to the Octet Rule. H only needs 2 valence electrons.
Be and B don’t need 8 valence electrons. S and P sometimes have more than 8 val.
Electrons. Lewis Structure Example: Methane (CH4) (1) Methane (CH4): First count total number of valence electrons.
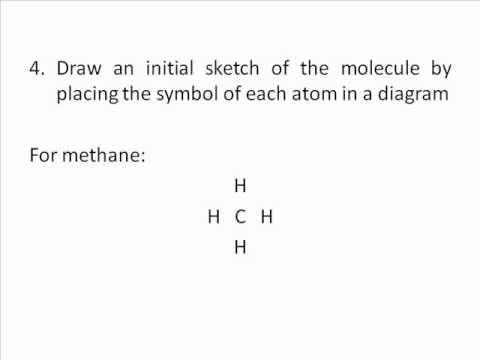
CH4 has 8 total valence electrons. Carbon has 4 and each H has 1 (total 4).
-Carbon typically like to make 4 bonds because Carbon has 4 valence e- and it . Drawing the Lewis Structure for CH 4.
For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org’s one of the easier Lewis structures to draw.
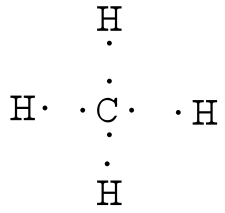
Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Dr.
B. explains how to draw the Lewis dot structure for CH 4 (methane).
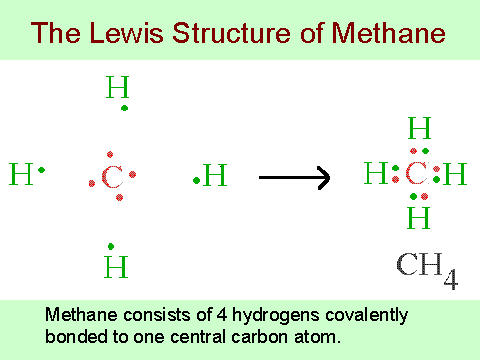
The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure.
This is because they can share a maximum of two electrons.Draw electron dot structure of methane and ethane – schematron.orgWhat is the Lewis dot structure of methane