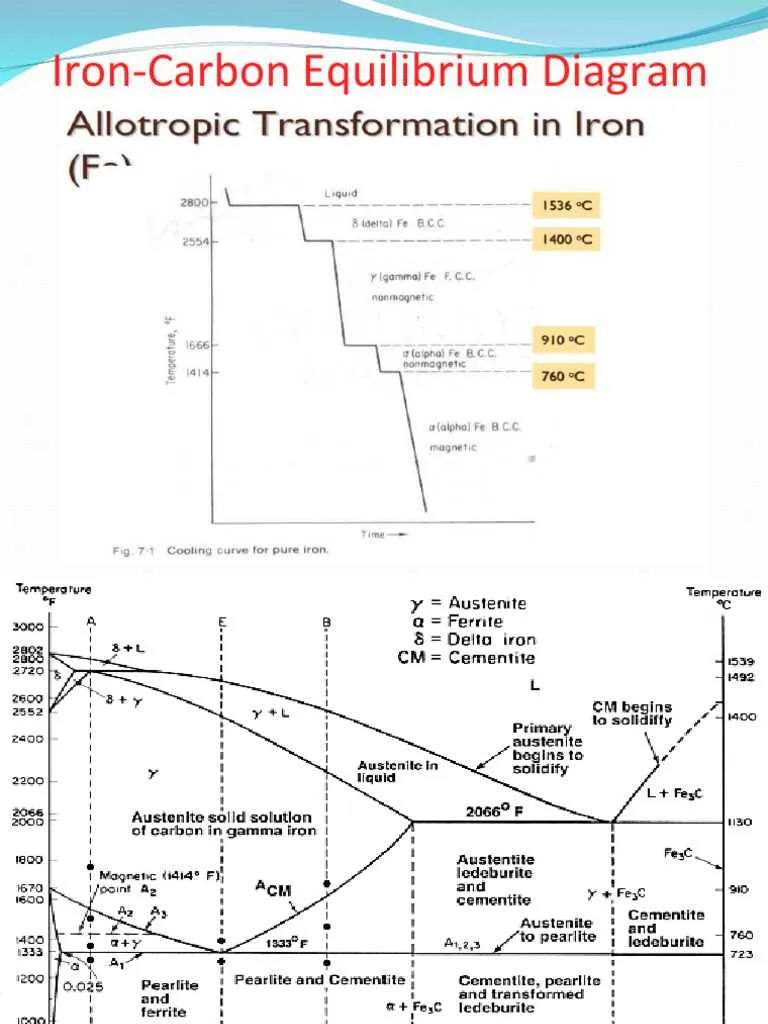
The study of phase transitions in steel alloys is essential for controlling material properties. To optimize processing, it is crucial to understand the temperature-pressure relationships that dictate the formation of different solid phases. This involves identifying regions of stable phases and the boundaries at which transformations occur under varying conditions of heat and pressure.
One must focus on critical points such as the eutectoid temperature, where a single phase undergoes a transformation into two distinct solid phases. Recognizing these boundaries allows for precise control over the microstructure, which directly influences strength, toughness, and ductility in processed materials. For example, controlling cooling rates through these transformation zones can lead to desired material characteristics.
Additionally, the solubility of elements in different phases varies with temperature. Knowledge of these solubility limits aids in designing alloys with improved performance. During alloying, it’s important to avoid exceeding the peritectic point, where the phase changes significantly, potentially compromising material integrity.
Phase Transformation in Steel Alloys
For the accurate prediction of phase transitions in steel mixtures, it is crucial to understand the behavior of the phases when varying temperature and composition. The phase boundaries, which separate different solid and liquid states, provide insight into the solubility limits of elements in the host metal. The formation of solid solutions, eutectic points, and other critical phases are key to tailoring material properties such as strength, hardness, and ductility.
Key Points to Consider:
- The α phase, also known as ferrite, is a solid solution of one element in another, typically stable at lower temperatures.
- With increasing temperature, the γ phase or austenite forms, which is crucial for processes like quenching and tempering.
- The cementite phase forms at higher concentrations of the solute, influencing the hardness and brittleness of the material.
- The eutectoid point marks the specific composition where two phases coexist in a balanced structure, significantly affecting the mechanical properties of alloys.
The manipulation of temperature and composition near these boundaries allows engineers to produce specific microstructures that optimize performance for different applications, from automotive components to structural materials.
Recommendations: For precise material control, monitor the transition temperatures and compositions carefully, and adjust heat treatment parameters accordingly to achieve desired material characteristics.
Understanding the Phases in the Iron-Carbon Phase Diagram
The phase transitions observed in this binary system can be divided into distinct regions that reflect various compositions and temperatures. Each phase corresponds to a unique microstructure with different mechanical properties. The key phases to consider are:
Ferrite: Present at lower temperatures and compositions, this phase is a solid solution of a minimal quantity of alloying elements in a body-centered cubic (BCC) structure. It is relatively soft and ductile, commonly observed in steels with low carbon content, below approximately 0.022% by weight.
Austenite: This phase exists in the high-temperature range and is a face-centered cubic (FCC) structure. It can dissolve significantly more alloying elements, contributing to greater strength. This phase is critical in heat treatment processes like quenching and tempering, with a critical temperature of around 727°C, where steel becomes entirely austenitic above this threshold.
Cementite: A compound that forms when higher concentrations of the alloying element are present. Cementite, with the formula Fe₃C, is an intermetallic phase that significantly enhances hardness and brittleness. It appears at compositions around 6.7% of the alloying element.
Pearlite: A two-phase structure composed of alternating layers of ferrite and cementite, formed through slow cooling. It provides a balance of strength and ductility, commonly seen in steels with medium carbon content (around 0.8% by weight).
Bainite: Formed at temperatures between those for pearlite and martensite, this phase consists of a mixture of ferrite and cementite in a fine, needle-like structure. It offers a combination of high strength and toughness, making it suitable for structural applications.
Martensite: A hard, supersaturated phase that forms during rapid cooling (quenching) from the austenite region. It has a body-centered tetragonal (BCT) structure, characterized by high hardness and brittleness. The carbon content influences the formation of martensite, with higher contents leading to greater hardness.
By understanding these phases, one can predict the mechanical properties of the alloy depending on its composition and thermal history. The transformation between these phases is crucial for manipulating materials to achieve specific characteristics such as hardness, strength, or ductility.
Implications for Steel Heat Treatment
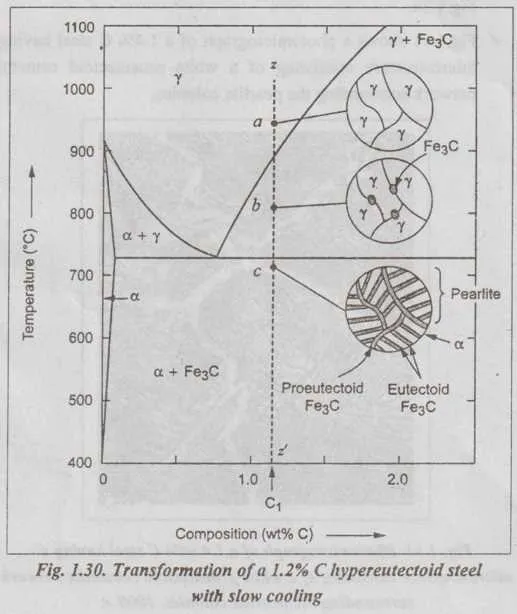
For optimal results in steel processing, understanding phase transitions is crucial. Tempering, quenching, and annealing processes directly relate to the temperature range where different solid phases form and dissolve. When heating alloys to specific points, it’s vital to ensure the alloy’s composition remains stable to avoid unwanted transformations.
In the case of austenite formation, temperatures above 723°C are required to achieve a fully austenitic structure. Rapid cooling from this phase leads to martensite formation, a hard and brittle structure. To mitigate brittleness, tempering at temperatures below 650°C allows controlled reorganization of the microstructure, enhancing toughness without significantly sacrificing strength.
Heat treatment also plays a key role in controlling the fraction of pearlite or bainite in the material. The temperature at which cooling occurs significantly influences which phase predominates. Slow cooling favors pearlite, while faster cooling rates promote bainite, offering different mechanical properties suitable for various applications.
When selecting processing parameters, it’s essential to recognize the impact of specific alloy contents, as small adjustments in composition shift phase boundaries. For example, higher amounts of alloying elements such as manganese or chromium will alter critical points, necessitating adjustments in temperature control during treatment cycles.
In conclusion, mastery of temperature control during heat treatment based on phase transformation knowledge ensures the production of steel with tailored mechanical properties, from hardness to ductility. The precise regulation of heating and cooling steps is critical for achieving desired performance characteristics in final products.
How to Predict Microstructure Changes in Iron-Carbon Alloys
To predict microstructure variations in ferrous alloys, focus on the key phase transformations and composition-dependent behavior. Begin by analyzing the cooling rate, as it influences the formation of phases such as pearlite, bainite, and martensite.
- Identify the alloy’s composition and temperature range. The eutectoid composition (0.8% carbon) is crucial in determining phase transitions.
- Assess the cooling rate: Slow cooling promotes the formation of pearlite, while rapid cooling leads to martensite.
- Use lever rule calculations to estimate the phase fractions. This provides insight into the relative amounts of phases at specific temperatures.
For alloys containing high levels of carbon, examine the carbon concentration in the solid phase and liquid phase. The solubility limit of carbon in the solid phase decreases as temperature drops.
- At temperatures above the eutectoid point, ferrite and austenite coexist in different ratios, depending on the temperature.
- Below the eutectoid temperature, the transformation to pearlite or bainite can occur, based on cooling conditions.
The key to understanding microstructure is knowing the effect of the alloy’s composition on phase boundaries. Austenite stability and its transformation into martensite or ferrite are pivotal.
- Calculate the fraction of austenite that will transform into martensite when quenched.
- Identify the critical cooling rate to achieve a fully martensitic structure, which is important for high-strength applications.
For high-strength low-alloy steels, focus on refining the cooling rate during the heat treatment process to achieve desired microstructures.