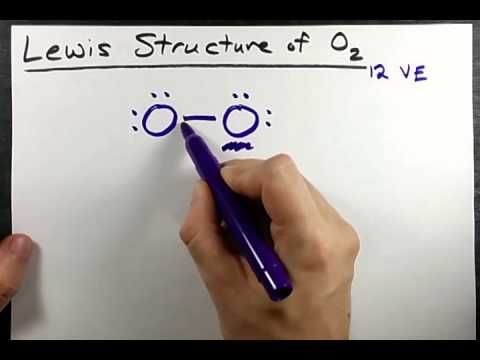
Lewis Structures for O2.
Step-by-step tutorial for drawing the Lewis Structure for O2. The covalent bond in an oxygen molecule, O 2 (oxygen gas) is non-polar – electrons are shared equally.
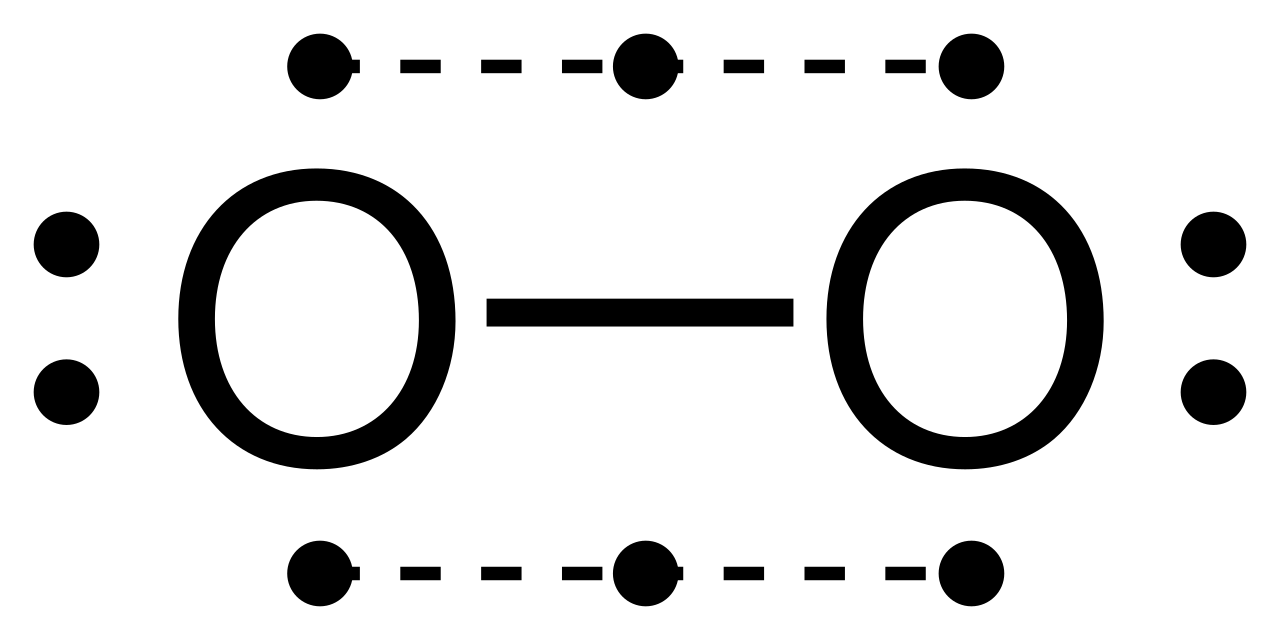
Draw the Lewis dot structure for each. Now, this is only one way we can draw the electron dot diagram for Oxygen.
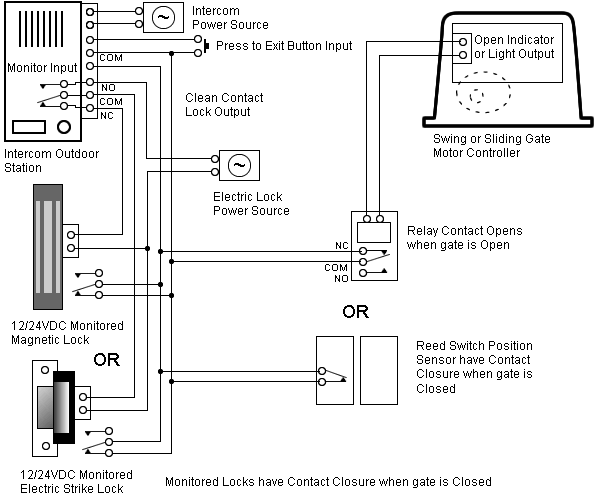
So as you may of remember from Chemistry class, before it can pair up on any other . Answer to (a) Construct a Lewis structure for O2 in which each atom achieves an octet of electrons.
(b) Explain (b) Explain why it is necessary to form a double bond in the Lewis structure. (c) The bond in Step 1 of 4. (A) Lewis structure of -.
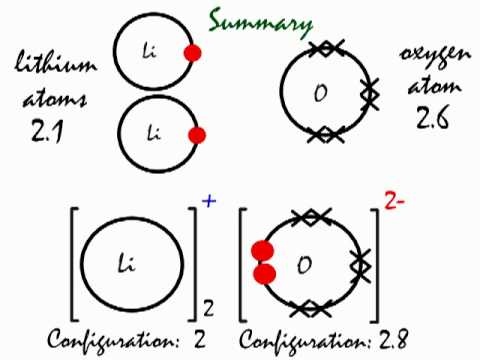
Oxygen atoms have 6 valence electrons. They need a stable octet but forming one bond wouldn’t help as this would leave them with only seven and one of.In nature, the Lewis structure for oxygen exists this way so it could react with other elements and in nature, it is a gas so it exists as O2.
As you can see, O2, each oxygen is connected by a single bond, each gets two octets, and on the bottom of each O, each O has one unpaired electron. Drawing the Lewis Structure for O 2 (Dioxygen or Oxygen Gas).
Oxygen (O 2) is a commonly tested Lewis structure due to it’s importance on schematron.org also is a good example of a molecule with a double bond. There are 12 valence electrons available for the Lewis structure for O Video: Drawing the Lewis Structure for O 2. Lewis structures extend the concept of the electron dot diagram by adding lines between atoms to represent shared pairs in a chemical bond.
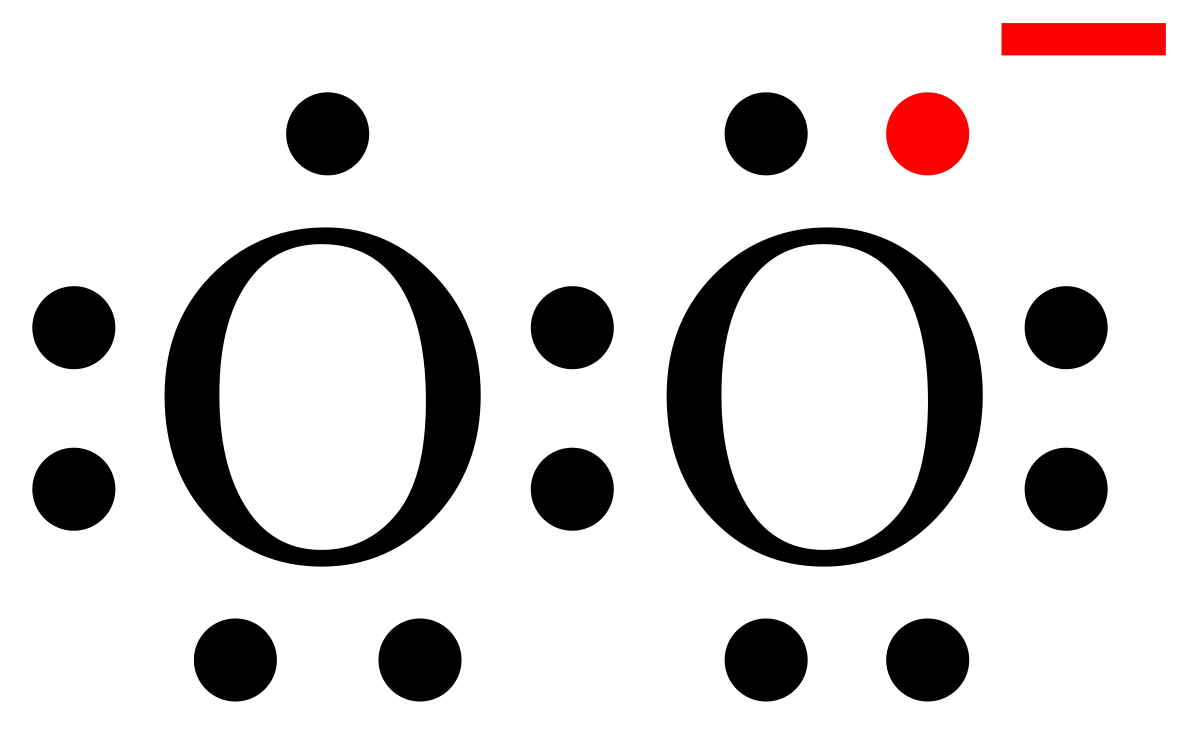
Lewis structures show each atom and its position in the structure of the molecule using its chemical symbol. Transcript: This is the O2 2- Lewis structure.
For the peroxide ion, Oxygen has six valence electrons. We have two Oxygens, and then we need to take in account these extra two valence electrons up here, so we’ll just add them for a total of 14 valence electrons for the 02 2- Lewis structure.
Transcript: OK, we’re going to do the Lewis dot structure for O2. Let’s start. Looking on the periodic table, we can find Oxygen in group 6 or 16, and that means it has 6 valence electrons.
But we have two of them so we’ll multiply that by 2.What is the electron dot diagram for an oxygen atom? | SocraticWhat is the Lewis Structure of O2