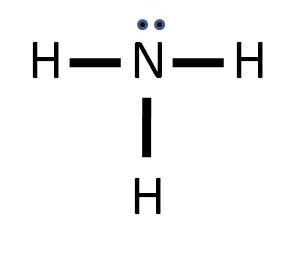
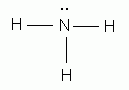
Electron Dot Structure of NH3 by Jeff Bradbury – February 17, – Lewis Electron Dot Structure for ammonia molecule NH3.
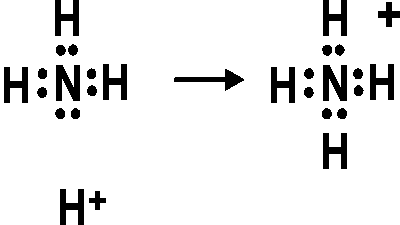
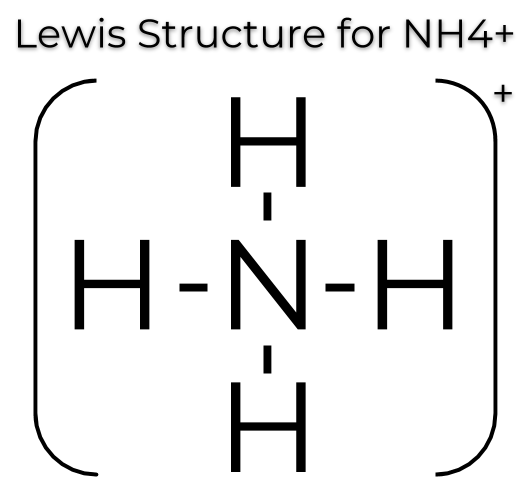
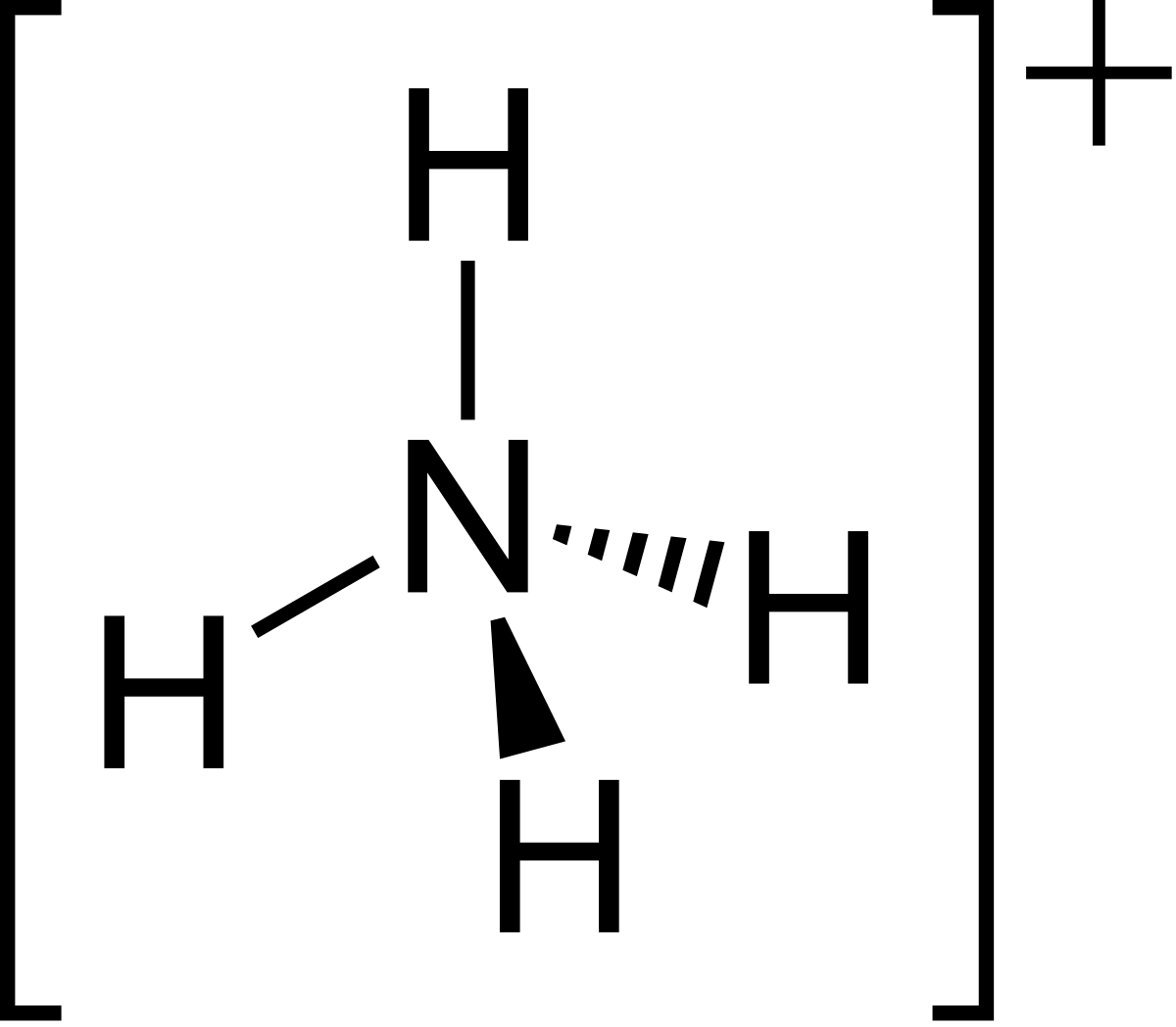
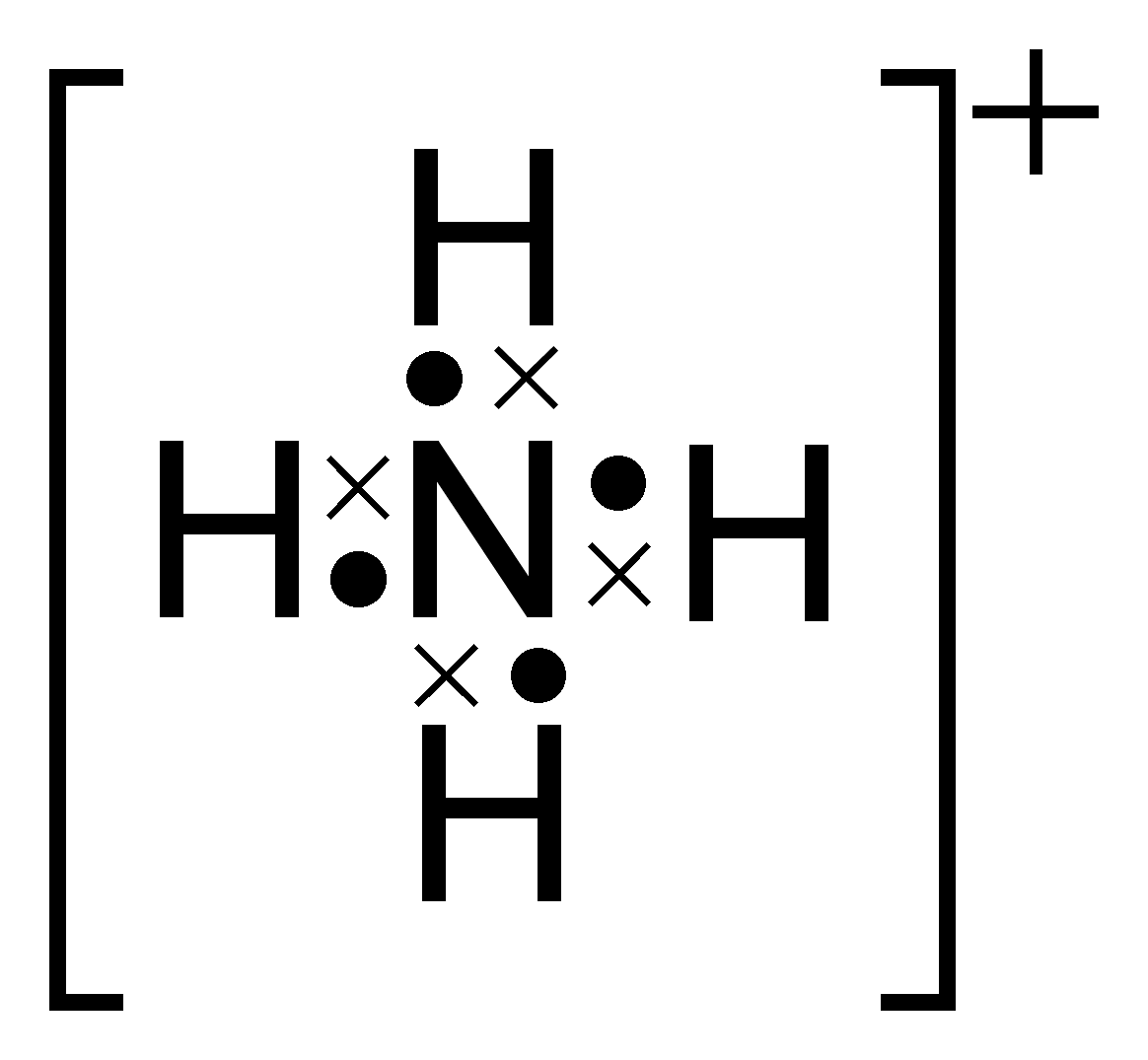
ShowMe is an open learning community featuring interactive lessons on a variety of topics. The Lewis Dot Structure for NH4+ (Ammonium) is shown above.
These kinds of structures can also be shown by representing each of the bonds with two dots. A step-by-step explanation of how to write the Lewis Dot Structure for NH3 ( Ammonia or Nitrogen Trihydride).
The Lewis structure for NH3 is. There are 8 valence electrons to distribute .. And 3xxN-H bonds, and one nitrogen-centred lone pair of electrons.Get the free “Lewis structure” widget for your website, blog, WordPress, Blogger, or iGoogle.
Find more Chemistry widgets in Wolfram|Alpha. Ammonia (NH 3) is a commonly tested Lewis structure due to it’s widespread use in agriculture as a fertilizer.
It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3.
Use information from step 4 and 5 to draw the lewis structure. Nitrogen goes in the centre.
Lewis dot structure of ammonia. Alternatively a dot method can be used to draw the lewis structure of NH3.
Calculate the total valence electrons in NH3 molecule. N=5,H=1×3=3 Total=8 Put Nitrogen in the center and three hydrogen atoms on the sides.
Lewis Dot Structures (2): Water and Ammonia
Drawing the Lewis Structure for NH 3. Viewing Notes: NH 3 (Ammonia) is a commonly tested Lewis structure.
It’s not particularly difficult but is an important structure. In the NH 3 Lewis structure (and all structures), hydrogen goes on the outside.
Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. Lewis Structure (electron dot diagram) for ammonia OR. Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone .The Lewis Dot Structure for NH4+ – MakeTheBrainHappyLewis Dot Structures (2): Water and Ammonia | Janet Gray Coonce