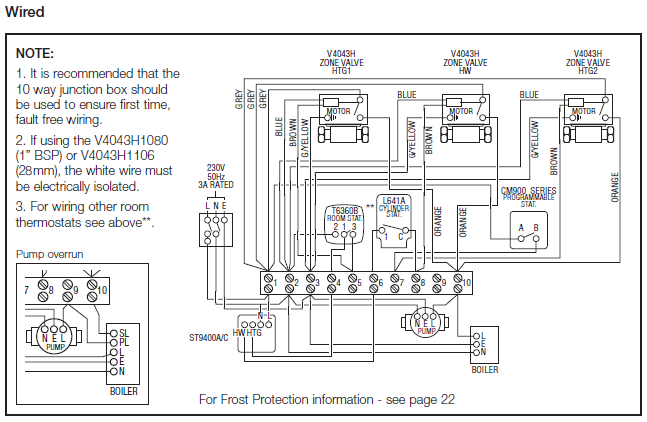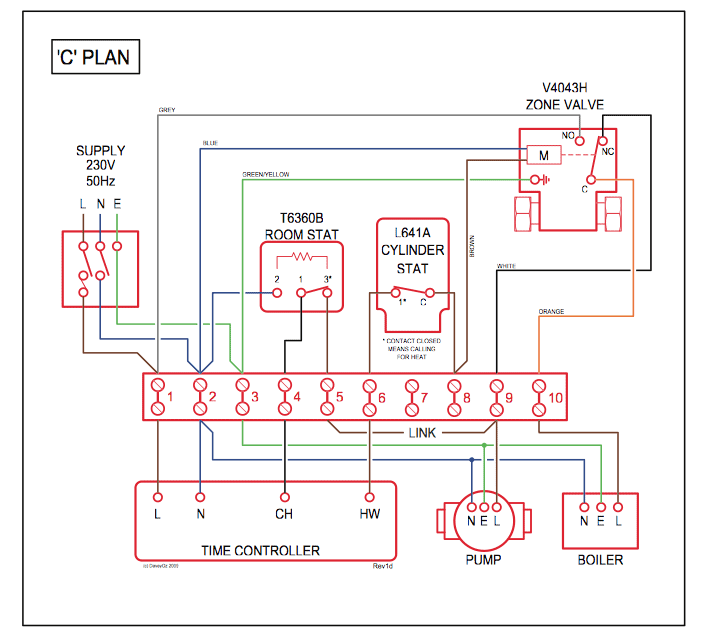
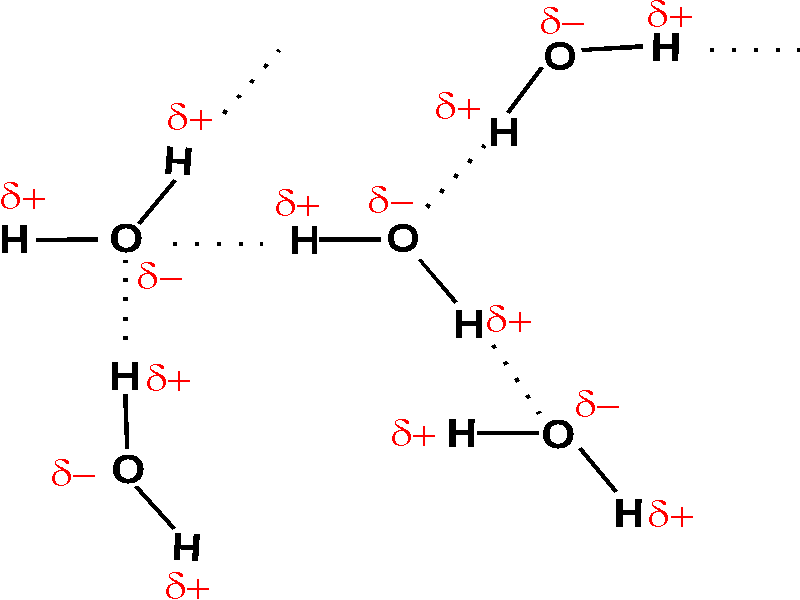
(c) The electrostatic potential diagram of the water molecule. Figure The polarity of the N—H bonds occurs because nitrogen has a greater electronegativity than hydrogen. (b) The dipole moment of the ammonia molecule oriented in an electric field.
The same type of behavior is observed for the NH3 molecule (Fig. You can tell that this atom belongs to the element _____ because . The ammonia molecule in the diagram has the observed bond orientation because N has.
(c) The electrostatic potential diagram of the water molecule. Figure The polarity of the N—H bonds occurs because nitrogen has a greater electronegativity than hydrogen.
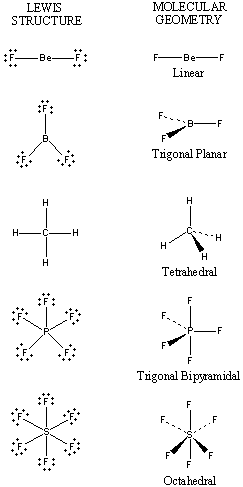
(b) The dipole moment of the ammonia molecule oriented in an electric field. The same type of behavior is observed for the NH3 molecule (Fig.
the energy that matter possess because of its location or structure .. The ammonia molecule in the diagram has the observed bond orientation because a. (c) The electrostatic potential diagram of the water molecule. The polarity of the NOH bonds occurs because nitrogen has a greater electronegativity than hydrogen.
(b) The dipole moment of the ammonia molecule oriented in an electric field. ❯ Bond Polarity and Dipole Moments We have seen that when hydrogen.Part F The ammonia molecule in the diagram has the observed bond orientation because All of the above. Part G Without making or breaking bonds, the pictured molecule can change its shape because rotation can occur around single bonds%(11).
The ammonia molecule in the diagram has the observed bond orientation because from BIO at Western Michigan University. The ammonia molecule in the diagram has the observed bond orientation because All of the above (N has 7 protons in its nucleus.
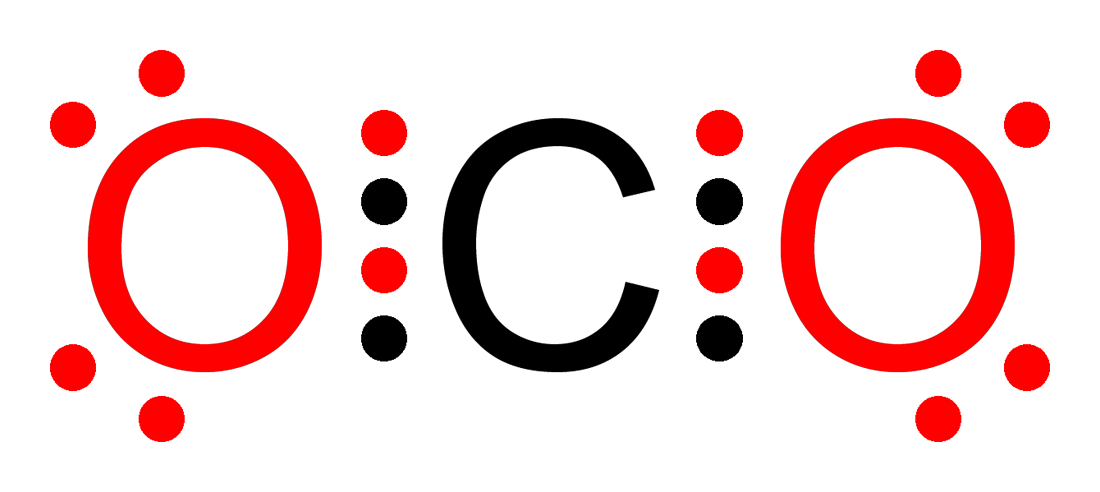
Since experimental evidence indicates that this molecule is bent (bond angle º) and has equal length sulfur: oxygen bonds ( Å), a single formula is inadequate, and the actual structure resembles an average of the two formulas. chapter 2 question set 2 KEY CONCEPTS: • Matter consists of chemical elements in pure form and in combinations called compounds.
• An element’s properties depend on the structure of its atoms. Without making or breaking bonds, the pictured molecule can change its shape because rotation can occur around single bonds. Two C atoms form a double bond.CH Chapter 4 – Covalent Bonds and Molecular Compounds – ChemistryAmmonia Molecule | Shape | TutorVista