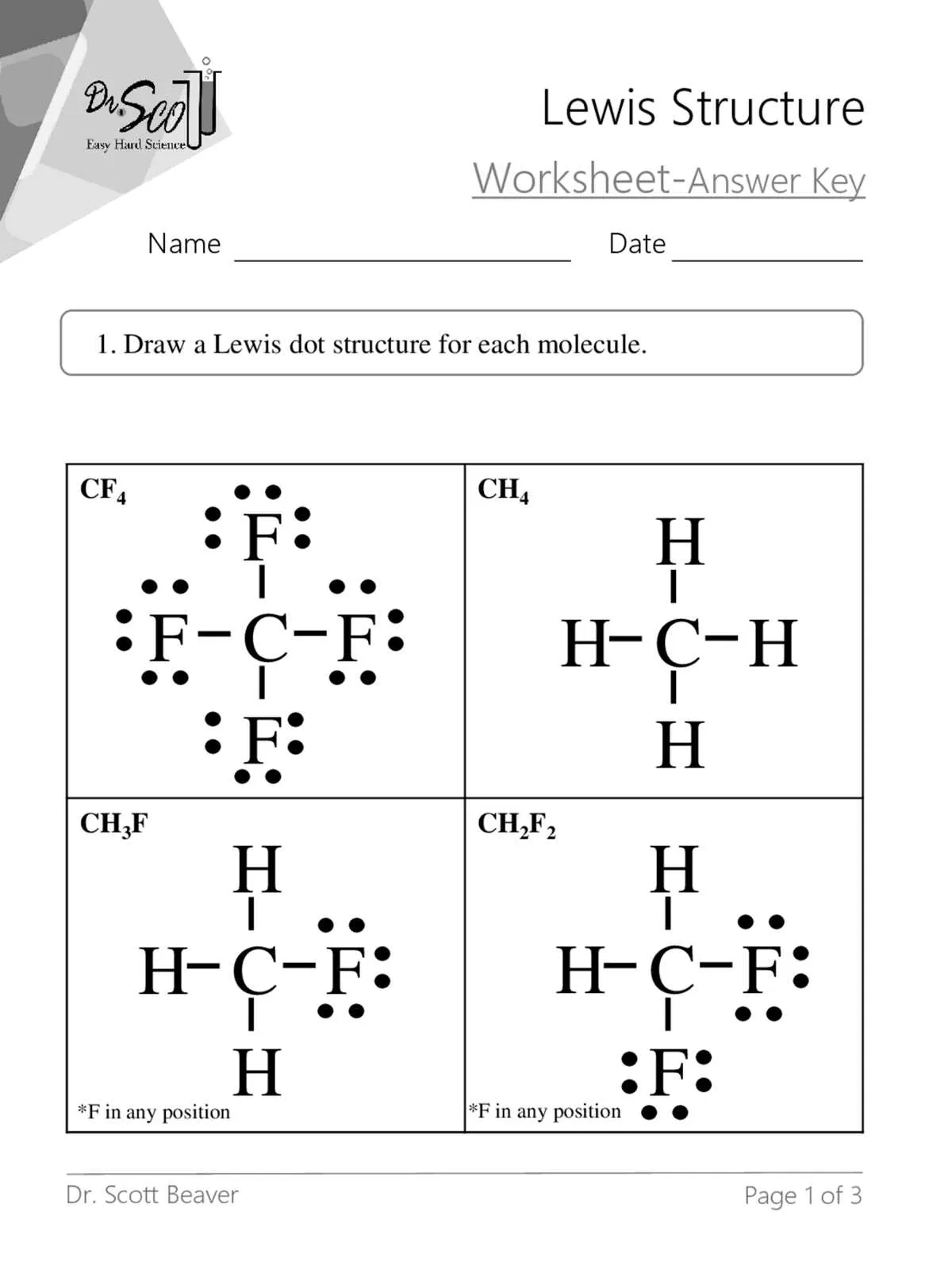
Begin by clearly identifying the valence electrons of each atom involved in the molecule. This is the foundation for building any accurate bonding structure. For a more precise understanding, visualize the electrons as pairs or individual dots around the symbol of the atom, following the rules of electron pairing. Be sure to consider the octet rule for most elements, ensuring that atoms tend to form bonds that result in eight electrons surrounding them when possible.
For effective electron placement: Start with the least electronegative atom at the center. Surround it with other atoms by connecting them with pairs of electrons, forming single bonds initially. Then, attempt to create double or triple bonds if necessary to satisfy the octet rule for each atom involved. Pay close attention to the fact that some elements, like hydrogen, only require two electrons to complete their shell.
Finally, always review the number of electrons in the structure to ensure that the total matches the valence electrons available from all atoms in the molecule. Once this is done, evaluate the formal charges for each atom to determine if the structure is the most stable configuration. Adjust as necessary, and remember that the goal is to create a stable arrangement of electrons that mirrors the actual atomic interactions.
Mastering Electron Representation
Start by ensuring each element’s valence electrons are correctly counted. Identify the number of electrons in the outermost shell, as this will determine bonding potential. For example, elements in group 1 have one electron available for bonding, while group 7 elements have seven. Draw the atom symbol at the center and place dots or other symbols around it to represent these electrons. Pay close attention to how the electrons are distributed, ensuring they follow the octet rule where applicable, filling the outer shell to a stable configuration. Make use of pairings to show shared electrons in covalent bonds, ensuring clarity in the depiction of molecular interactions.
When constructing these models, avoid overcrowding; distribute electrons evenly around the atom. For molecules with multiple atoms, repeat this process for each element, considering both lone pairs and shared electrons. For ions, adjust the total number of electrons to reflect the charge. Remember that the structure of larger molecules or more complex compounds may require extra steps to visualize electron sharing or lone pairs more clearly.
Practice with different elements and compounds to improve accuracy and speed. For example, when modeling a simple molecule like H2O, place two pairs of electrons on oxygen and one on each hydrogen, ensuring the bonds are represented by shared electrons. Over time, aim for consistency in placement and understanding of how different atoms interact electronically. This approach will help when predicting molecular shapes and reactions.
Step-by-Step Guide to Drawing Molecular Electron Structures
Start by identifying the total number of valence electrons available from all atoms in the molecule. This is key for accurate placement of electrons around atoms.
Determine the central atom. This is typically the atom that can form the most bonds and is often less electronegative than others.
Place single bonds between the central atom and surrounding atoms. Each bond represents two electrons, so subtract the electrons used for bonding from the total count.
Distribute the remaining electrons around the outer atoms, filling their valence shells to a maximum of 8 electrons (octet rule), if applicable.
If there are still electrons left after filling outer atoms, place them on the central atom to form lone pairs. If the central atom doesn’t have an octet, consider forming double or triple bonds with surrounding atoms to complete the structure.
Finally, check for formal charges. If possible, rearrange electrons to minimize charges on atoms, ensuring that the structure follows the most stable configuration.
Common Mistakes When Drawing Electron Structures and How to Avoid Them

One frequent error is neglecting to account for the octet rule. Many molecules require a full set of valence electrons around atoms, especially for carbon, nitrogen, oxygen, and halogens. Double-check the number of electrons each atom should have and ensure all atoms are correctly paired.
- Incorrect Electron Pairing: Ensure that each bond between atoms consists of shared pairs of electrons. Inadequate or overestimated sharing can lead to incorrect representations. Always count each electron and remember that bonds are formed by electron pairs.
- Forgetting Formal Charges: Atoms should have formal charges calculated correctly. Incorrect formal charge assignment can lead to structural errors. When assigning formal charges, consider the number of valence electrons each atom should have and the number of electrons in the structure.
- Misplacing Lone Pairs: Ensure that non-bonding electrons are placed properly around the atoms. These lone pairs must not be skipped or placed in incorrect locations, which could affect the molecular geometry.
Avoid assumptions about atomic connectivity. Misidentifying central atoms or improper bonding between atoms can lead to misrepresented structures. Always consider electronegativity and atom size to identify which atoms should be connected.
- Bonding Order Errors: Be cautious with bond order. Some molecules may require multiple bonds (double or triple), and it’s important to reflect this accurately. For example, oxygen in O2 has a double bond, and nitrogen in N2 forms a triple bond.
- Skipping Expanded Octet: Elements in period 3 and beyond (like phosphorus and sulfur) can hold more than eight electrons in their valence shells. Don’t forget that these atoms can have expanded octets when necessary.
Finally, verify the total number of valence electrons. The sum of electrons in all bonds and lone pairs must equal the total number of valence electrons from the atoms involved. Double-check to avoid errors in electron count.
Understanding Electron Pairing and Octet Rule in Molecular Structures
Start by recognizing that each atom aims to complete its outer electron shell by sharing, gaining, or losing electrons. This is critical when determining how atoms bond with one another. Focus on the number of valence electrons, as this dictates how they pair up with electrons from neighboring atoms.
Atoms follow the octet rule, which states that atoms typically bond to attain eight electrons in their outer shell, resembling the electron configuration of noble gases. When forming bonds, atoms pair their unpaired electrons to create stable electron configurations. For example, in the case of oxygen, which has six valence electrons, it pairs two electrons from another atom to fill its shell with a total of eight electrons.
In molecules with multiple bonds, such as double or triple bonds, electron sharing is maximized to satisfy the octet rule for all involved atoms. Ensure that the total number of electrons in the structure accounts for the sum of the valence electrons from all atoms in the molecule. For molecules like CO₂, the central atom (carbon) shares two pairs of electrons with each oxygen atom, completing their octets.
Pay close attention to atoms like hydrogen and helium, which follow the duet rule instead of the octet rule, aiming for two electrons in their outer shell. This is especially important when determining bonding for smaller molecules like H₂ or He. Consider how electron pairs are distributed and ensure that each atom’s valence shell is satisfied according to its specific requirements.