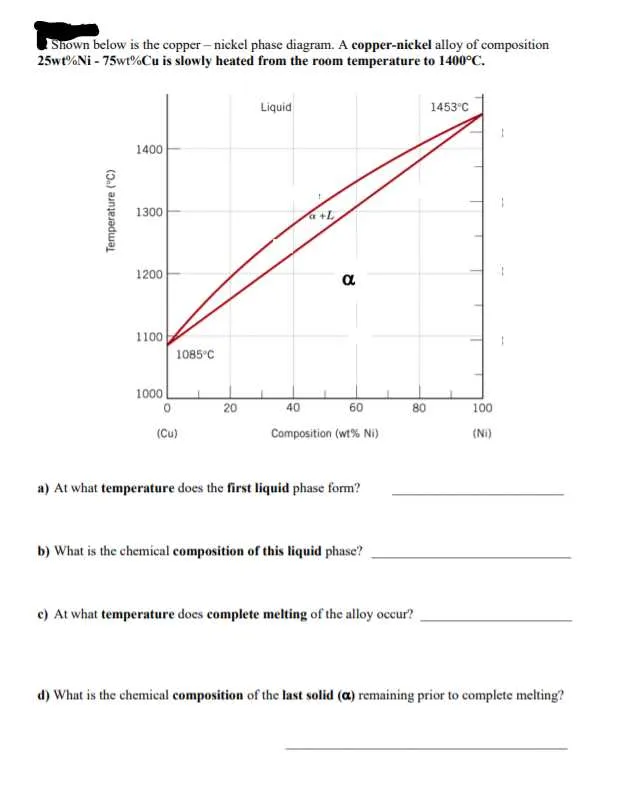
To analyze the behavior of two specific metals under varying thermal conditions, focus on their mutual solubility limits. For best results, consult the equilibrium curves that highlight liquid and solid phases in response to temperature and composition changes. These curves will indicate where solid and liquid coexist at particular proportions of each element.
Key parameters include the critical temperature at which complete fusion occurs, and the solidification sequence, which can be significantly influenced by the ratio of the two metals. Pay close attention to the eutectic composition, where the lowest melting point occurs, offering insights into optimal alloying strategies.
In practice, such an equilibrium chart reveals important information about the temperature ranges where one phase fully transforms into another, essential for manufacturing alloys with precise mechanical properties. Ensure accurate testing by measuring the system’s reaction at different compositions to achieve desired phase transitions.
Detailed Plan for Copper Nickel Phase Diagram Article
Begin by focusing on the binary alloy system that includes two key metallic elements, detailing their melting points, solubility ranges, and phase transformations. Provide a thorough analysis of the equilibrium between the solid and liquid states, including liquidus and solidus lines for varying compositions. Highlight how temperature and concentration influence the formation of intermediate phases, and examine the regions where distinct crystalline structures form.
Describe the critical points in the system, including eutectic points, peritectic points, and the overall temperature-composition relationship. Specify the methods used for constructing the equilibrium curves and the challenges involved in obtaining precise data, including the impact of experimental conditions on results.
Provide insights into the alloy’s mechanical properties, such as hardness, tensile strength, and corrosion resistance, as they change across the various phases. Discuss how alloying elements impact these properties and what is most important when designing materials for specific industrial applications.
Conclude by referencing the importance of this binary system in practical metallurgy, focusing on how knowledge of the composition and temperature relationships aids in material selection and performance optimization for high-temperature and structural applications.
Understanding the Phase Diagram of Copper-Nickel Alloys
To accurately interpret the relationship between composition and temperature in these materials, focus on the solubility limits and phase transitions. For a binary mixture, the solidus and liquidus lines are crucial, indicating the onset of solidification and complete liquefaction, respectively. Pay special attention to the eutectic point, where two solid phases coexist in equilibrium at a fixed temperature and composition.
The alloy system exhibits a complete solid solubility in the solid state, with a single-phase solid solution forming across the entire range of compositions. In contrast, the liquid phase shows limited solubility, with the maximum solubility of one element in the other decreasing as temperature drops.
At lower temperatures, there are regions where two distinct solid phases are stable, each with its own unique crystal structure. Identifying these two-phase zones is essential when designing for specific mechanical properties, as the combination of different phases can enhance strength and resistance to corrosion.
Furthermore, understanding the intermetallic compounds that form at certain compositions allows for optimization of alloy performance, especially in high-temperature environments. The presence of these compounds significantly influences the alloy’s thermal stability and mechanical behavior.
In practical applications, the cooling rate plays a vital role in the resulting microstructure. Rapid cooling can result in a fine-grained structure, whereas slow cooling allows for the formation of coarser phases, affecting material properties such as ductility and strength.
Impact of Temperature and Composition on Phase Transitions
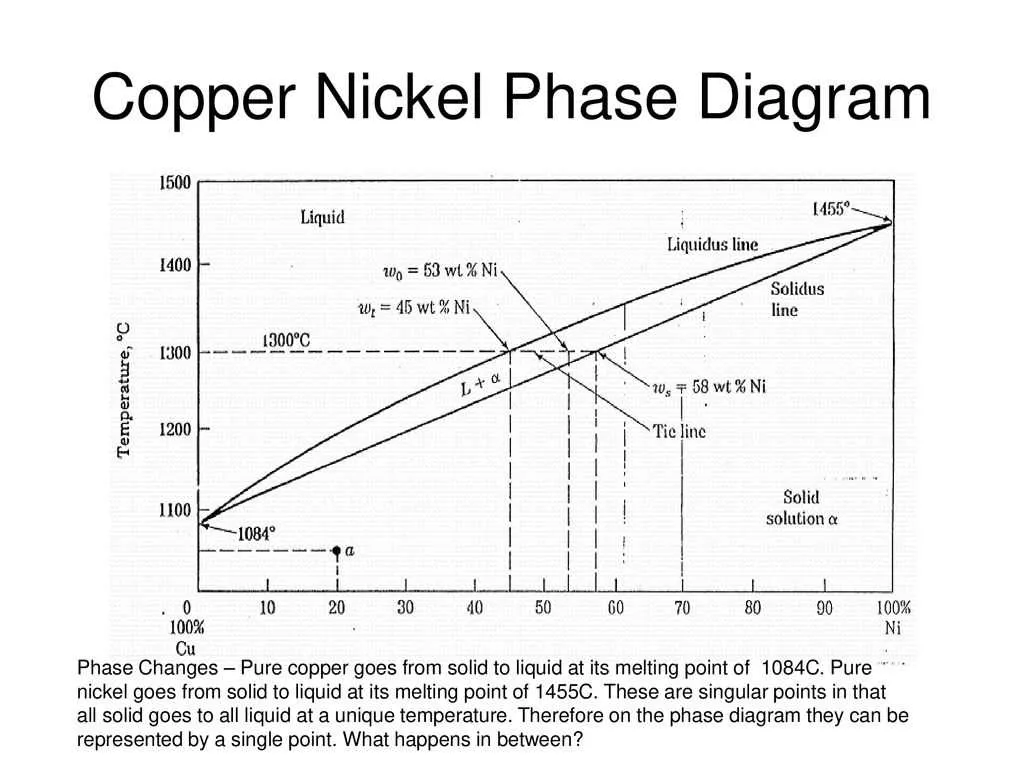
Temperature and composition significantly influence the behavior of alloys, determining the formation and transformation of different solid and liquid structures. The following factors should be carefully considered when analyzing transitions in metallic systems:
- Temperature: An increase in temperature generally promotes the melting of solid components, leading to the formation of a homogeneous liquid phase. As temperature decreases, solidification occurs, and different phases may separate, depending on the alloy’s composition.
- Composition: The ratio of the two metals in the mixture affects the melting points of the phases. For example, higher concentrations of one metal may shift the solidus and liquidus lines, modifying the temperature range for phase changes.
Key observations include:
- Solidus and Liquidus Boundaries: The temperature at which a solid starts to melt (solidus) and the temperature at which the last remaining solid becomes liquid (liquidus) depend on the mixture’s composition. As the proportion of one metal increases, the solidus and liquidus temperatures may shift.
- Intermediate Phases: Certain compositions will lead to the formation of intermediate solid phases, which can affect the alloy’s mechanical and thermal properties.
Optimizing temperature and composition during the manufacturing process is crucial for achieving the desired material properties, especially in industrial applications requiring specific structural qualities.
Practical Applications of Copper-Nickel Phase Diagram in Material Engineering
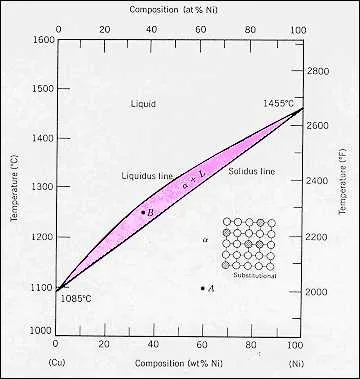
Engineers can utilize the phase transitions between two metals to optimize alloy composition for specific applications. By studying the equilibrium curves, it becomes possible to control mechanical properties such as hardness, tensile strength, and corrosion resistance. Understanding the eutectic composition allows for the production of materials with superior durability under harsh environmental conditions, such as marine or high-temperature environments.
In manufacturing, precise control over cooling rates is essential to achieve desired microstructure. This approach is particularly useful in the aerospace and automotive industries, where performance under stress is critical. By adjusting the ratio of the two elements, one can fine-tune the alloy to achieve higher oxidation resistance or improved wear properties, thus extending the lifespan of critical components.
Furthermore, knowledge of solidus and liquidus lines helps in refining welding techniques, ensuring strong bonds between metal parts. This is particularly important when joining metals of similar compositions, where a fine balance between melting points and solidification rates must be maintained for optimal joint strength.
Alloy development for specific engineering solutions can be accelerated through simulation tools that predict the behaviors of these materials under various conditions. This predictive capability saves time in the design and testing phases, allowing for the creation of high-performance materials suited to specialized applications such as heat exchangers or desalination plants.